
The addition of HBr to an alkene in the presence of peroxide is an example of:
A. Electrophilic addition reaction
B. Nucleophilic addition reaction
C. Free-radical addition reaction
D. Formation of carbocation as an intermediate
Answer
233.1k+ views
Hint: When hydrogen bromide is added to alkenes in presence of peroxide, it reacts with anti-Markovnikov’s addition mechanism. Peroxides form free radicals that initiate the reaction and add bromine radical at terminal carbon.
Complete step-by-step answer:
Peroxides have a weak oxygen-oxygen bond which on heating results in homolytic fragmentation of this bond i.e. the bond breaks in order to leave one unpaired electron on each atom involved in the reaction. Strong sources of light such as floodlight or other source of light radiation which reaches into the near UV might also serve to weaken this bond.
Only a catalytic amount of peroxide is needed to get the reaction started, although one molar equivalent of HBr is essentially required to result in complete addition of HBr to the alkene.
This results in a highly reactive alkoxy radical which then abstracts hydrogen from H-Br, releasing a bromine radical. The bromine radical is the one that is added to the alkene from the molecule hydrogen bromide.
Preferably addition to an alkene tends to occur in such a way that the most stable free radical is formed, tertiary radical here in HBr. That’s the reason that bromine ends up on the least substituted carbon of the alkene. This tertiary radical then eliminates hydrogen from H-Br, liberating a bromine radical, and this way the cycle continues.
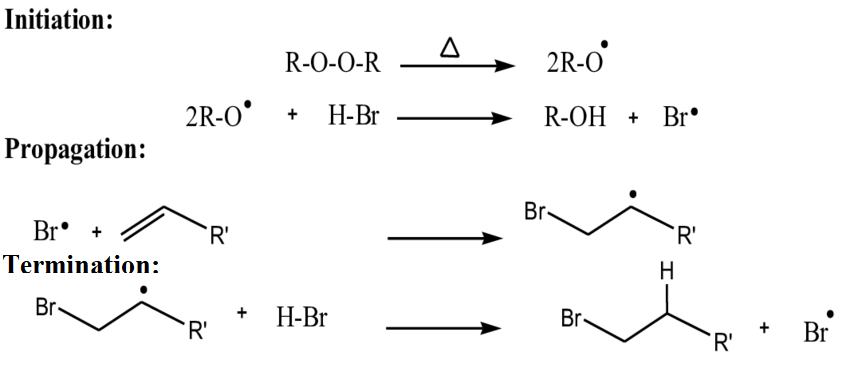
Hence, the correct option is (C).
Note: In absence of peroxide, if alkene reacts with hydrogen bromide, the attack of bromine is due to electrophilic addition reaction in which carbocation is formed as an intermediate. It is also called Markovnikov’s addition in which attack takes place on more substituted carbon.
Complete step-by-step answer:
Peroxides have a weak oxygen-oxygen bond which on heating results in homolytic fragmentation of this bond i.e. the bond breaks in order to leave one unpaired electron on each atom involved in the reaction. Strong sources of light such as floodlight or other source of light radiation which reaches into the near UV might also serve to weaken this bond.
Only a catalytic amount of peroxide is needed to get the reaction started, although one molar equivalent of HBr is essentially required to result in complete addition of HBr to the alkene.
This results in a highly reactive alkoxy radical which then abstracts hydrogen from H-Br, releasing a bromine radical. The bromine radical is the one that is added to the alkene from the molecule hydrogen bromide.
Preferably addition to an alkene tends to occur in such a way that the most stable free radical is formed, tertiary radical here in HBr. That’s the reason that bromine ends up on the least substituted carbon of the alkene. This tertiary radical then eliminates hydrogen from H-Br, liberating a bromine radical, and this way the cycle continues.

Hence, the correct option is (C).
Note: In absence of peroxide, if alkene reacts with hydrogen bromide, the attack of bromine is due to electrophilic addition reaction in which carbocation is formed as an intermediate. It is also called Markovnikov’s addition in which attack takes place on more substituted carbon.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




