
The angular shape of water molecule is due to:
(a).High electron affinity of oxygen
(b).very high repulsion between lone pair and bond pair
(c).very high repulsion between lone pair and lone pair
(d).small size of hydrogen
Answer
516.6k+ views
Hint: Shape of compounds depends on various factors such as availability of different orbitals, no of electron, lone pair, bond pair etc. Valence shell electron pair repulsion theory (VSEPR) helps us to predict the shape of different covalent compounds. It tells us about the repulsion of electrons in the valence shell.
Complete answer:
VSEPR theory gives us the information about lone pairs present in the valence shell, the unpaired electron forms a bond with another unpaired electron and the paired electrons are left as lone pairs. Which causes repulsion between bond pair-bond pair, lone pair-bond pair, lone pair-lone pair and they start arranging themselves to minimize the repulsion. During this process the geometry predicted by hybridization gets distorted and different geometry of molecules are seen.
The shape of the water molecule is angular due to the presence of two lone pairs on the oxygen atom.
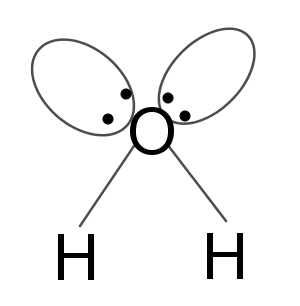
And the magnitude of repulsion follows:
\[l.p. - l.p. > l.p. - b.p. > b.p. - b.p.\]
As repulsion among lone pairs is highest the shape of the water molecule becomes V-shaped or angular.
Option (c) is the correct choice.
Option (b) is incorrect as repulsion is seen higher in b.p. then l.p. bond b.p.
Option (d) is incorrect as there is no role of size in the angular shape of the water molecule.
Option (a) is incorrect as electron affinity is the amount of energy released when an electron is added in a neutral atom.
Note:
When we predict the shape of a molecule the bonded pairs of a molecule are considered and not the lone pairs present on that molecule. The geometry of the molecule remains the same which is tetrahedral in case of water. Only the orientation of electron pairs in space changes which changes the shape of the molecule.
Complete answer:
VSEPR theory gives us the information about lone pairs present in the valence shell, the unpaired electron forms a bond with another unpaired electron and the paired electrons are left as lone pairs. Which causes repulsion between bond pair-bond pair, lone pair-bond pair, lone pair-lone pair and they start arranging themselves to minimize the repulsion. During this process the geometry predicted by hybridization gets distorted and different geometry of molecules are seen.
The shape of the water molecule is angular due to the presence of two lone pairs on the oxygen atom.

And the magnitude of repulsion follows:
\[l.p. - l.p. > l.p. - b.p. > b.p. - b.p.\]
As repulsion among lone pairs is highest the shape of the water molecule becomes V-shaped or angular.
Option (c) is the correct choice.
Option (b) is incorrect as repulsion is seen higher in b.p. then l.p. bond b.p.
Option (d) is incorrect as there is no role of size in the angular shape of the water molecule.
Option (a) is incorrect as electron affinity is the amount of energy released when an electron is added in a neutral atom.
Note:
When we predict the shape of a molecule the bonded pairs of a molecule are considered and not the lone pairs present on that molecule. The geometry of the molecule remains the same which is tetrahedral in case of water. Only the orientation of electron pairs in space changes which changes the shape of the molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




