
The atomic number of an element having the valence shell electronic configuration \[4{{s}^{2}}4{{p}^{6}}\] is:
(A) 35
(B) 36
(C) 37
(D) 38
Answer
566.7k+ views
Hint: Valence shell configuration \[4{{s}^{2}}4{{p}^{6}}\] suggests that it is the electronic configuration of a noble gas element because valence shell is containing 8 electrons. Also \[4{{s}^{2}}4{{p}^{6}}\] suggests that it is the element of the 4th period, so we can conclude that this is a noble gas element of the 4th period.
complete answer:
We know that in a neutral atom the number of protons is always equal to the number of neutrons, therefore the atomic number of the neutral atom will be equal to the number of electrons.
Electron filling is done according to aufbau principle as shown in the diagram below:
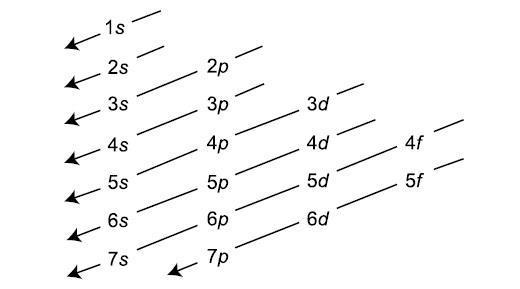
According to the above diagram, first energy level will have 2 electrons; second energy level will have 8 electrons; third energy level will be having 18 electrons along with 2 electrons those were filled in $4{{s}^{{}}}$ orbital before we started the filling in $3{{d}^{{}}}$ subshell. Now that takes the count of electrons to 30 and according to our desired electronic configuration we need to have $4{{s}^{2}}4{{p}^{6}}$ electronic configuration in the valence shell.
So we need to fill 6 more electrons in the valence shell and according to the aufbau principle they will be filled in $4p$ subshell that will give us the desired electronic configuration $4{{s}^{2}}4{{p}^{6}}$ valence shell.
Now the total electrons we have filled are the addition of 30 and 6 and that will be 36.
So the atomic number of the element will be 36.
Correct answer is Option (B)
Additional information: Krypton is a colorless, odorless gas. It has a boiling point of $-152.9{}^{0}C$ and a density of $3.64g{{L}^{-1}}$. That makes krypton about 2.8 times as dense as air.
Note: Shell number of valence shell always represents the period of the element. If an element is in the 3rd period, its outermost energy level will be 3rd and similarly for other elements as well. But always remember that f-block elements are exceptions and in transition elements electrons of d-subshell of penultimate orbit also take part in bond formation, therefore (n-1)d-electrons also act like valence electrons.
complete answer:
We know that in a neutral atom the number of protons is always equal to the number of neutrons, therefore the atomic number of the neutral atom will be equal to the number of electrons.
Electron filling is done according to aufbau principle as shown in the diagram below:

According to the above diagram, first energy level will have 2 electrons; second energy level will have 8 electrons; third energy level will be having 18 electrons along with 2 electrons those were filled in $4{{s}^{{}}}$ orbital before we started the filling in $3{{d}^{{}}}$ subshell. Now that takes the count of electrons to 30 and according to our desired electronic configuration we need to have $4{{s}^{2}}4{{p}^{6}}$ electronic configuration in the valence shell.
So we need to fill 6 more electrons in the valence shell and according to the aufbau principle they will be filled in $4p$ subshell that will give us the desired electronic configuration $4{{s}^{2}}4{{p}^{6}}$ valence shell.
Now the total electrons we have filled are the addition of 30 and 6 and that will be 36.
So the atomic number of the element will be 36.
Correct answer is Option (B)
Additional information: Krypton is a colorless, odorless gas. It has a boiling point of $-152.9{}^{0}C$ and a density of $3.64g{{L}^{-1}}$. That makes krypton about 2.8 times as dense as air.
Note: Shell number of valence shell always represents the period of the element. If an element is in the 3rd period, its outermost energy level will be 3rd and similarly for other elements as well. But always remember that f-block elements are exceptions and in transition elements electrons of d-subshell of penultimate orbit also take part in bond formation, therefore (n-1)d-electrons also act like valence electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




