
The best reagent for converting, \[2 - \]phenylpropanamide into $1 - $phenylethanamine is:
$A)\dfrac{{NaOH}}{{B{r_2}}}$
$B)\dfrac{{LiAl{H_4}}}{{ether}}$
$C)\dfrac{{NaB{H_4}}}{{Methanol}}$
$D)excess\dfrac{{{H_2}}}{{Pt}}$
Answer
519.3k+ views
Hint: To find how to convert $2 - $phenylpropanamide into $1 - $phenylethanamine, we must find the type of reaction. The type of reaction that will take place is Hoffmann bromamide reaction.
Complete answer:
The reaction will take place by using Hofmann degradation reaction.
Hoffmann bromamide reaction is when an amide is treated with bromine in a liquid or ethanolic arrangement of sodium hydroxide, degradation of amide happens prompting the development of ${1^ \circ }$ amine. This reaction includes degradation of amide and is prevalently known as Hoffmann bromamide degradation reaction.
This happens due to intramolecular shifting of the alkyl group.
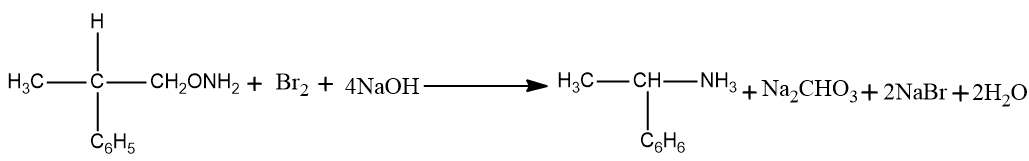
– The hydroxide part of the solid base attacks the amide. The amide is presently deprotonated prompting the arrangement of water and the anion of the amide.
– The anion presently attacks the diatomic bromine in an alpha replacement response. The bromine-bromine bond breaks and N-Bromamide is framed alongside Br-anion.
– The N-Bromamide is presently attacked again by the base, prompting its deprotonation and the development of water alongside the age of bromamide anion.
-which is fortified with the carbonyl carbon presently bonds with the nitrogen all things considered. All the while, the bromide anion leaves the compound. This prompts the arrangement of an isocyanate.
– The expansion of water to the isocyanate prompts the development of carbamic corrosive in a nucleophilic expansion response.
– The carbamic corrosive presently loses carbon dioxide, giving a contrarily charged nitrogen attached to one hydrogen and the ethyl bunch. At the point when this is protonated by the water, the necessary essential amine is framed.
Therefore, option (A) is the correct answer.
Note:
Hoffman bromamide reaction is one the major reaction for the synthesis of primary amines and methylamines and aniline. It is also used to prepare anthranilic acid.
Complete answer:
The reaction will take place by using Hofmann degradation reaction.
Hoffmann bromamide reaction is when an amide is treated with bromine in a liquid or ethanolic arrangement of sodium hydroxide, degradation of amide happens prompting the development of ${1^ \circ }$ amine. This reaction includes degradation of amide and is prevalently known as Hoffmann bromamide degradation reaction.
This happens due to intramolecular shifting of the alkyl group.

– The hydroxide part of the solid base attacks the amide. The amide is presently deprotonated prompting the arrangement of water and the anion of the amide.
– The anion presently attacks the diatomic bromine in an alpha replacement response. The bromine-bromine bond breaks and N-Bromamide is framed alongside Br-anion.
– The N-Bromamide is presently attacked again by the base, prompting its deprotonation and the development of water alongside the age of bromamide anion.
-which is fortified with the carbonyl carbon presently bonds with the nitrogen all things considered. All the while, the bromide anion leaves the compound. This prompts the arrangement of an isocyanate.
– The expansion of water to the isocyanate prompts the development of carbamic corrosive in a nucleophilic expansion response.
– The carbamic corrosive presently loses carbon dioxide, giving a contrarily charged nitrogen attached to one hydrogen and the ethyl bunch. At the point when this is protonated by the water, the necessary essential amine is framed.
Therefore, option (A) is the correct answer.
Note:
Hoffman bromamide reaction is one the major reaction for the synthesis of primary amines and methylamines and aniline. It is also used to prepare anthranilic acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




