
The Birch reduction of toluene gives:
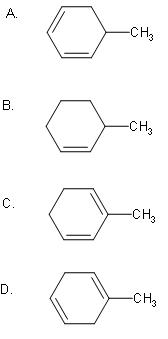

Answer
585.9k+ views
Hint: For identifying the correct product of the toluene firstly we have to study about the Birch reduction and then we have to write the whole reaction of toluene (Benzene with methyl group) that undergoes Birch reduction.
Complete step by step answer:
- In the given questions, we have to identify the correct product which is formed when the toluene undergoes Birch reduction.
- As we know that the reduction is the process in which the addition of the hydrogen atom and removal of the oxygen atom takes place.
- In the Birch reduction, the aromatic ring the methyl group is also known as toluene, in it the double bond breaks and the attachment of hydrogen take place.
- The chemical reaction will be:
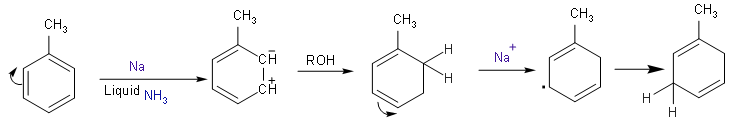
- Here, the reduction is also known as 1, 4 reductions because the reduction or addition of hydrogen at the first and fourth carbon atom takes place.
- The reaction takes place in the presence of sodium or lithium metal and liquid ammonia in the presence of an alcohol such as ethanol.
- At the end as we can see that the formation of unconjugated cyclohexadiene takes place because the single and double bonds are not present in an alternate manner.
So, the correct answer is “Option D”.
Note: While reducing the compound with the Birch reduction method the temperature is usually kept low so that the liquid ammonia can be used as a solvent. The method is very useful when only one double bond is required to break.
Complete step by step answer:
- In the given questions, we have to identify the correct product which is formed when the toluene undergoes Birch reduction.
- As we know that the reduction is the process in which the addition of the hydrogen atom and removal of the oxygen atom takes place.
- In the Birch reduction, the aromatic ring the methyl group is also known as toluene, in it the double bond breaks and the attachment of hydrogen take place.
- The chemical reaction will be:

- Here, the reduction is also known as 1, 4 reductions because the reduction or addition of hydrogen at the first and fourth carbon atom takes place.
- The reaction takes place in the presence of sodium or lithium metal and liquid ammonia in the presence of an alcohol such as ethanol.
- At the end as we can see that the formation of unconjugated cyclohexadiene takes place because the single and double bonds are not present in an alternate manner.
So, the correct answer is “Option D”.
Note: While reducing the compound with the Birch reduction method the temperature is usually kept low so that the liquid ammonia can be used as a solvent. The method is very useful when only one double bond is required to break.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




