
The bond angle of ammonia is ${109^o}{28'}$ .
A. True
B. False
Answer
584.7k+ views
Hint: According to the valence bond theory, there exist three $N - H$ bonds in the structure of ammonia and they are at equal angles from each other in the trigonal pyramid geometry. But after the evolution of valence shell electron pair repulsion theory (VSEPR), the lone pair of nitrogen atoms is also considered to be a part of the geometry of the ammonia structure which provides equal repulsion to all the $N - H$ bonds.
Complete step by step answer:
According to the valence shell electron pair repulsion theory, the structure of ammonia can be drawn as:
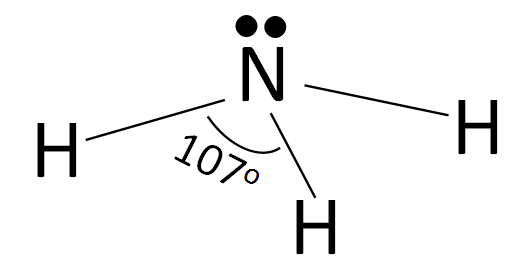
According to the structure of ammonia shown above, we can clearly see that the electron lone pair on the nitrogen atom provides repulsion to the $N - H$ bonds equally. The hybridization of $N{H_3}$ is $s{p^3}$ and according to that the geometry should have been tetrahedral in case of ammonia and the bond angle should have been ${109^o}{28'}$ which is true in the case of methane. But here, in the case of ammonia, the bond angle decreases from ${109^o}{28'}$ to ${107^o}$ and the geometry changes from tetrahedral to trigonal pyramidal with the lone pair of electrons at the top and the rest of the $N - H$ bonds below it.
Thus the correct option is B. False.
Note:
To know about the hybridization of ammonia we need to look at the regions around the nitrogen atom. Around the nitrogen atom, there is a lone pair of electrons and 3 sigma bonds which are formed between the nitrogen and hydrogen atoms. Thus, these four regions make ammonia $s{p^3}$ hybridized because we have 1‘s’ and three ‘p’ orbitals, that are being hybridized around the nitrogen atom. The hydrogen atoms have s orbitals which will overlap with the 3 ‘p’ orbitals of nitrogen. That is the hybridization of $N{H_3}$ .
Complete step by step answer:
According to the valence shell electron pair repulsion theory, the structure of ammonia can be drawn as:

According to the structure of ammonia shown above, we can clearly see that the electron lone pair on the nitrogen atom provides repulsion to the $N - H$ bonds equally. The hybridization of $N{H_3}$ is $s{p^3}$ and according to that the geometry should have been tetrahedral in case of ammonia and the bond angle should have been ${109^o}{28'}$ which is true in the case of methane. But here, in the case of ammonia, the bond angle decreases from ${109^o}{28'}$ to ${107^o}$ and the geometry changes from tetrahedral to trigonal pyramidal with the lone pair of electrons at the top and the rest of the $N - H$ bonds below it.
Thus the correct option is B. False.
Note:
To know about the hybridization of ammonia we need to look at the regions around the nitrogen atom. Around the nitrogen atom, there is a lone pair of electrons and 3 sigma bonds which are formed between the nitrogen and hydrogen atoms. Thus, these four regions make ammonia $s{p^3}$ hybridized because we have 1‘s’ and three ‘p’ orbitals, that are being hybridized around the nitrogen atom. The hydrogen atoms have s orbitals which will overlap with the 3 ‘p’ orbitals of nitrogen. That is the hybridization of $N{H_3}$ .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




