
The bonds present in the ethylene molecule are:
A.2 sigma 2 pi
B.5 sigma 1 pi
C.4 sigma 2 pi
D.3 sigma 2 pi
Answer
582.3k+ views
Hint: Basically, the sigma and pi bond are the types of covalent bond that differ in the overlapping of atomic orbitals. The sigma bonds are a result of head to head overlapping of atomic orbitals whereas pi bonds are formed by the lateral overlapping of two atomic orbitals.
Complete step by step answer:
Generally, sigma and pi bonds are a type of covalent bond. Various bond parameters such as bond length, bond angle and bond enthalpy depend on the way the overlapping of atomic orbital takes place. Now this overlap occurs in two major ways and thus gives rise to two types of covalent bond known as sigma and pi bond. Let’s discuss both the bonds.
Now, the first one is the sigma bond. This type of bond is formed by head-on positive overlap of atomic orbitals along the internuclear axis. Moreover, the electrons participating in this type of bonds are known as sigma electrons. Let’s see some of the examples of sigma bonds.
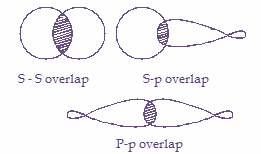
1. \[S - S\] overlapping- In this kind of overlapping, one ‘s’ orbital from each participating atom undergoes head-on overlapping along the internuclear axis. In this case the s-orbital must be half filled before it overlaps with another. For example, ${H_2}$ molecule.
2. \[S - P\] overlapping- In this kind of overlapping, one half filled s orbital overlaps with one half-filled p orbital. For example, $N{H_3}$ molecule.
3. \[P - P\] overlapping- In this case, one half filled p orbital from each participating atom undergoes head-on overlapping along the internuclear axis. For example, $C{l_2}$ molecule.
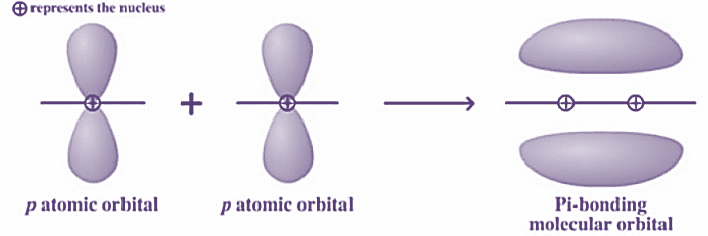
Now, pi bonds are formed by the sideways overlap of the atomic orbitals along a direction perpendicular to the internuclear axis. These bonds are generally weaker than sigma bonds. So, basically during the form of pi bonds, the axes of the atomic orbitals are parallel to each other whereas the overlapping is perpendicular to the internuclear axis.
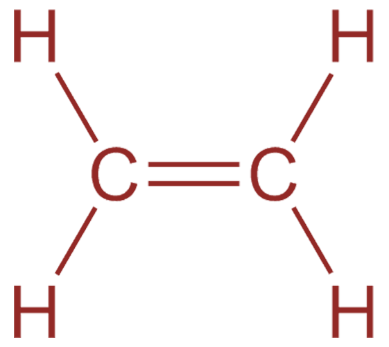
Now, in the case of ethylene molecules $({H_2}C = C{H_2})$ , there are double bonds between two carbon atoms. One sigma bond and one pi bond. Moreover, each carbon has two sigma bonds with two hydrogen atoms as carbon has valency four. Therefore, ethylene has five sigma bonds and one pi bond.
Hence, option B is correct.
Note:Single bonds are always sigma bonds. Moreover, a triple bond consists of two pi bonds and one sigma bond and double bond contains one sigma and one pi bond. The combination of sigma and pi bonds is always stronger than a single sigma bond.
Complete step by step answer:
Generally, sigma and pi bonds are a type of covalent bond. Various bond parameters such as bond length, bond angle and bond enthalpy depend on the way the overlapping of atomic orbital takes place. Now this overlap occurs in two major ways and thus gives rise to two types of covalent bond known as sigma and pi bond. Let’s discuss both the bonds.
Now, the first one is the sigma bond. This type of bond is formed by head-on positive overlap of atomic orbitals along the internuclear axis. Moreover, the electrons participating in this type of bonds are known as sigma electrons. Let’s see some of the examples of sigma bonds.
1. \[S - S\] overlapping- In this kind of overlapping, one ‘s’ orbital from each participating atom undergoes head-on overlapping along the internuclear axis. In this case the s-orbital must be half filled before it overlaps with another. For example, ${H_2}$ molecule.
2. \[S - P\] overlapping- In this kind of overlapping, one half filled s orbital overlaps with one half-filled p orbital. For example, $N{H_3}$ molecule.
3. \[P - P\] overlapping- In this case, one half filled p orbital from each participating atom undergoes head-on overlapping along the internuclear axis. For example, $C{l_2}$ molecule.

Now, pi bonds are formed by the sideways overlap of the atomic orbitals along a direction perpendicular to the internuclear axis. These bonds are generally weaker than sigma bonds. So, basically during the form of pi bonds, the axes of the atomic orbitals are parallel to each other whereas the overlapping is perpendicular to the internuclear axis.

Now, in the case of ethylene molecules $({H_2}C = C{H_2})$ , there are double bonds between two carbon atoms. One sigma bond and one pi bond. Moreover, each carbon has two sigma bonds with two hydrogen atoms as carbon has valency four. Therefore, ethylene has five sigma bonds and one pi bond.

Hence, option B is correct.
Note:Single bonds are always sigma bonds. Moreover, a triple bond consists of two pi bonds and one sigma bond and double bond contains one sigma and one pi bond. The combination of sigma and pi bonds is always stronger than a single sigma bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




