
The carbide of Lithium has the formula:
A.$LiC$
B.$L{i_2}C$
C.$L{i_4}C$
D.$L{i_2}{C_2}$
Answer
573.9k+ views
Hint:The compounds that consist of carbon and less electronegative elements and that are distinguished by their chemical bonding (ionic or covalent) are known as carbides. They are generally prepared at high temperatures.
Complete step by step answer:
Carbides are compounds that consist of carbon and less electronegative elements.
Carbides are usually prepared at very high temperatures from metals and metal oxides by combining metal with carbon.
They are extremely stable and have high melting points.
Lithium carbide:
a)Lithium carbide consists of lithium atom bonding to carbon carbon triple bond.
b)The structure of lithium carbide is as follows:
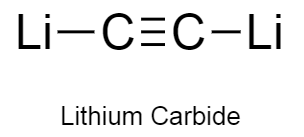
a)It is also known as dilithium acetylide.
b)It was first produced by Mossan.
c)It is insoluble in organic solvents.
d)It was first produced using lithium carbonate in the reaction given below:
$L{i_2}C{O_3} + 4C \to L{i_2}{C_2} + 3CO$
In this reaction when lithium carbonate reacts with carbon atoms it forms lithium carbide along with the carbon monoxide gas.
a)It is also prepared from various other reactions. The reactions are as follows:
1.When lithium hydride reacts with graphite it forms lithium carbide in the reaction given below:
$2LiH + 4C \to L{i_2}{C_2} + {C_2}{H_2}$
2.When metallic lithium reacts with ethylene in the atmosphere it produces lithium carbide.
$6Li + {C_2}{H_4} \to L{i_2}{C_2} + 4LiH$
Uses of Lithium Carbide:
-It is used in the carbon dating procedures.
So the correct answer is option D i.e $L{i_2}{C_2}$ .
Note:
-In lithium carbide, carbon-carbon atoms have a triple bond .
-Lithium carbide is often confused with lithium carbonate because of the similarity in their name and some of the properties. Also it has similar properties as of calcium carbide and is the only stable alkali metal that reacts with the graphite.
Complete step by step answer:
Carbides are compounds that consist of carbon and less electronegative elements.
Carbides are usually prepared at very high temperatures from metals and metal oxides by combining metal with carbon.
They are extremely stable and have high melting points.
Lithium carbide:
a)Lithium carbide consists of lithium atom bonding to carbon carbon triple bond.
b)The structure of lithium carbide is as follows:
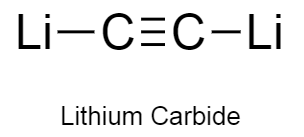
a)It is also known as dilithium acetylide.
b)It was first produced by Mossan.
c)It is insoluble in organic solvents.
d)It was first produced using lithium carbonate in the reaction given below:
$L{i_2}C{O_3} + 4C \to L{i_2}{C_2} + 3CO$
In this reaction when lithium carbonate reacts with carbon atoms it forms lithium carbide along with the carbon monoxide gas.
a)It is also prepared from various other reactions. The reactions are as follows:
1.When lithium hydride reacts with graphite it forms lithium carbide in the reaction given below:
$2LiH + 4C \to L{i_2}{C_2} + {C_2}{H_2}$
2.When metallic lithium reacts with ethylene in the atmosphere it produces lithium carbide.
$6Li + {C_2}{H_4} \to L{i_2}{C_2} + 4LiH$
Uses of Lithium Carbide:
-It is used in the carbon dating procedures.
So the correct answer is option D i.e $L{i_2}{C_2}$ .
Note:
-In lithium carbide, carbon-carbon atoms have a triple bond .
-Lithium carbide is often confused with lithium carbonate because of the similarity in their name and some of the properties. Also it has similar properties as of calcium carbide and is the only stable alkali metal that reacts with the graphite.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




