
The cell reaction involving the quinhydrone electrode is given below. What will be the electrode potential at \[pH = 3\] ?

Answer
502.5k+ views
Hint: The quinhydrone electrode is a special electrode used to measure the hydrogen ion concentration and pH of a particular solution. The half-cell reaction involving the quinhydrone electrode itself involves the hydrogen ions and is therefore used for the purpose of determining the pH of solutions.
Complete answer:
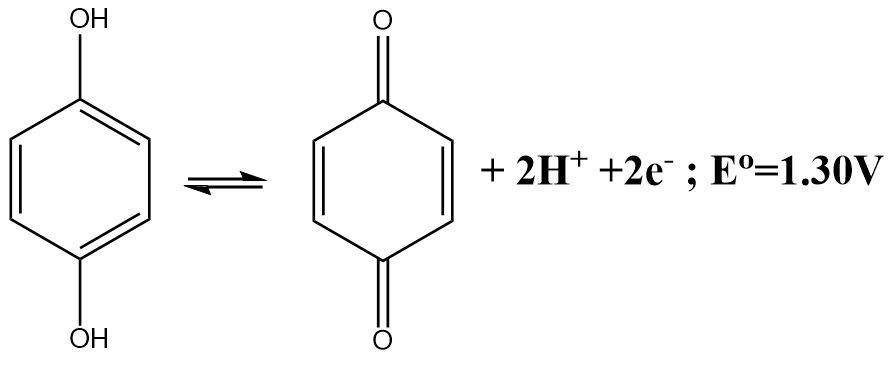
The molecular crystal formed between the Quinone \[(Q)\] and hydroquinone \[(Q{H_2})\] is known as quinhydrone. The dissolution of quinhydrone crystals into water initiates the decomposition process and the molecule breaks down into its constituent fragments and one of these constituents are the hydrogen ions.
The electrode contains a golf wire in the spiraled form that is dipped into the solution of unknown hydrogen ion concentration or the solution whose pH needs to be determined.
The electrode potential of any cell can be expressed in the terms of its standard electrode potential \[({E^ \circ })\] and the concentrations of the reactants and the products.
The half-cell reaction of a quinhydrone electrode indicates the fact that a Quinone molecule accepts two electrons and gets reduced in the process. The reaction can be written as follows:
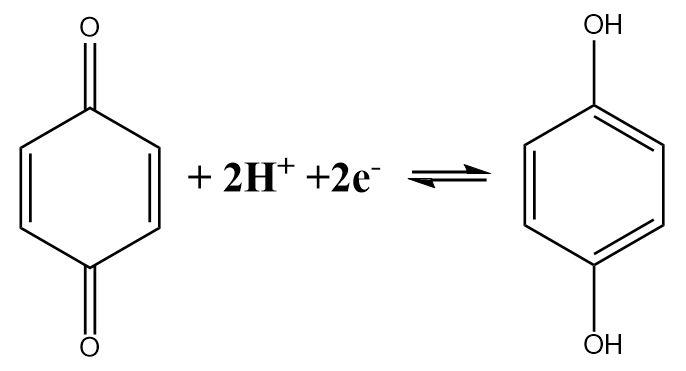
The expression for the reduction potential can be given as follows:
\[E = {E^ \circ } - \dfrac{{2.303RT}}{{2F}}\log \dfrac{{[Q{H_2}]}}{{[Q]{{[{H^ + }]}^2}}}\]
Which can be written as,
\[E = {E^ \circ } - \dfrac{{2.303RT}}{{2F}}\log \dfrac{1}{{{{[{H^ + }]}^2}}}\]
The negative log of concentration of hydrogen ions is nothing but pH and therefore the expression can be rewritten as follows:
\[E = {E^ \circ } - \dfrac{{2.303RT}}{F}pH\]
Assuming that the temperature is room temperature and putting in the stand values of Faraday’s constant and universal gas constant we get,
\[E = {E^ \circ } - (0.0591 \times pH)\]
Since, the standard electrode potential is given to be \[1.30V\] and the pH is given to be three, these values can be inserted in the above formula to get the final electrode potential,
\[E = 1.1227V\]
Which can be approximated to,
\[E \approx 1.10V\]
Hence, option (c) is correct.
Note:
Since, the activities of Quinone and hydroquinone remain equal in the reaction, their concentration terms get cancelled in the expression of electrode potential calculations. The activity of hydrogen ions is assumed to be approximately equal to its concentration i.e. the activity coefficient is taken to be one for the simplification of calculations.
Complete answer:
The molecular crystal formed between the Quinone \[(Q)\] and hydroquinone \[(Q{H_2})\] is known as quinhydrone. The dissolution of quinhydrone crystals into water initiates the decomposition process and the molecule breaks down into its constituent fragments and one of these constituents are the hydrogen ions.
The electrode contains a golf wire in the spiraled form that is dipped into the solution of unknown hydrogen ion concentration or the solution whose pH needs to be determined.
The electrode potential of any cell can be expressed in the terms of its standard electrode potential \[({E^ \circ })\] and the concentrations of the reactants and the products.
The half-cell reaction of a quinhydrone electrode indicates the fact that a Quinone molecule accepts two electrons and gets reduced in the process. The reaction can be written as follows:
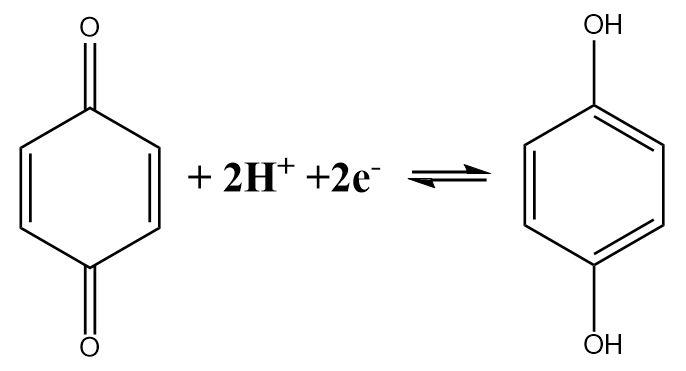
The expression for the reduction potential can be given as follows:
\[E = {E^ \circ } - \dfrac{{2.303RT}}{{2F}}\log \dfrac{{[Q{H_2}]}}{{[Q]{{[{H^ + }]}^2}}}\]
Which can be written as,
\[E = {E^ \circ } - \dfrac{{2.303RT}}{{2F}}\log \dfrac{1}{{{{[{H^ + }]}^2}}}\]
The negative log of concentration of hydrogen ions is nothing but pH and therefore the expression can be rewritten as follows:
\[E = {E^ \circ } - \dfrac{{2.303RT}}{F}pH\]
Assuming that the temperature is room temperature and putting in the stand values of Faraday’s constant and universal gas constant we get,
\[E = {E^ \circ } - (0.0591 \times pH)\]
Since, the standard electrode potential is given to be \[1.30V\] and the pH is given to be three, these values can be inserted in the above formula to get the final electrode potential,
\[E = 1.1227V\]
Which can be approximated to,
\[E \approx 1.10V\]
Hence, option (c) is correct.
Note:
Since, the activities of Quinone and hydroquinone remain equal in the reaction, their concentration terms get cancelled in the expression of electrode potential calculations. The activity of hydrogen ions is assumed to be approximately equal to its concentration i.e. the activity coefficient is taken to be one for the simplification of calculations.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




