
The conversation of m- nitrophenol to resorcinol involves _________ respectively.
(A) hydrolysis, diazotization and reduction
(B) diazotization, reduction and hydrolysis
(C) hydrolysis, reduction and diazotization
(D) reduction diazotization and hydrolysis
Answer
539.7k+ views
Hint :We know that the phenol is a natural substance as well as it can be manufactured. It is colourless to white solid when it is pure. The product of it is liquid. It has a sickeningly sweet and has distinct odour. It can evaporate more slowly than water. It is very flammable and this means it can catch fire. Phenol is used primarily in the production of phenolic resins and in the manufacture of nylon and other synthetic fibres.
Complete Step By Step Answer:
It can be used in slimicides which means that it can kill bacteria and fungi in slimes. It is also used in antiseptic and disinfectant. When we talk about mouthwashes we can see that there are medicinal preparations of the same.
When $ M- $ nitrophenol is heated, it decomposes to emit toxic fumes of oxides of nitrogen. It is seen as a crystalline material. Phenols are known to react as weak organic acids. The process consists of the process of reduction which is followed by diazotization. The product of diazotization then undergoes hydrolysis. The final product of the resorcinol.
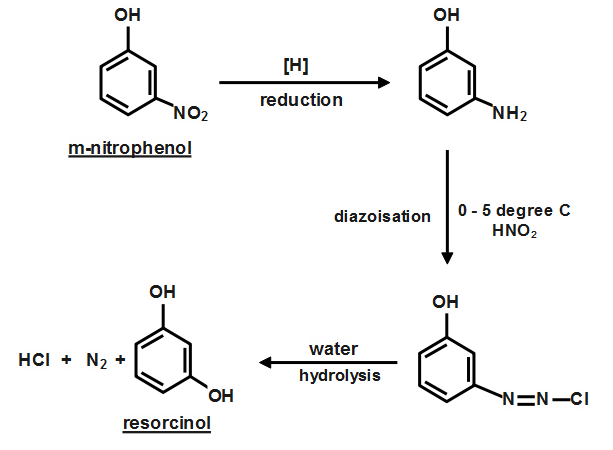
Therefore the correct answer is option (D), which is the conversation of m- nitrophenol to resorcinol involves reduction diazotization and hydrolysis respectively.
Note :
Note that the phenol is corrosive to skin but as it has anaesthetic qualities it will numb rather than burn. It can be seen that it may be lethal when skin absorption occurs. It has a role as a disinfectant, an antiseptic drug, a human xenobiotic metabolite and a mouse metabolite. It is a conjugate acid of a phenolate.
Complete Step By Step Answer:
It can be used in slimicides which means that it can kill bacteria and fungi in slimes. It is also used in antiseptic and disinfectant. When we talk about mouthwashes we can see that there are medicinal preparations of the same.
When $ M- $ nitrophenol is heated, it decomposes to emit toxic fumes of oxides of nitrogen. It is seen as a crystalline material. Phenols are known to react as weak organic acids. The process consists of the process of reduction which is followed by diazotization. The product of diazotization then undergoes hydrolysis. The final product of the resorcinol.

Therefore the correct answer is option (D), which is the conversation of m- nitrophenol to resorcinol involves reduction diazotization and hydrolysis respectively.
Note :
Note that the phenol is corrosive to skin but as it has anaesthetic qualities it will numb rather than burn. It can be seen that it may be lethal when skin absorption occurs. It has a role as a disinfectant, an antiseptic drug, a human xenobiotic metabolite and a mouse metabolite. It is a conjugate acid of a phenolate.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




