
The conversion of Benzaldehyde into benzyl alcohol takes place by:
(A)Fitting reaction
(B) Wurtz-fittig reaction
(C)Wurtz reaction
(D)Cannizzaro’s reaction
Answer
585.9k+ views
Hint:Benzaldehyde is an organic compound with formula ${C_7}{H_6}O$. It is the simplest aromatic aldehyde and one of the most industrially useful. It is a colourless liquid with an almond like odor. Benzaldehyde does not contain any $\alpha $-hydrogen and thus can undergo cannizzaro reaction easily.
Complete step by step answer:
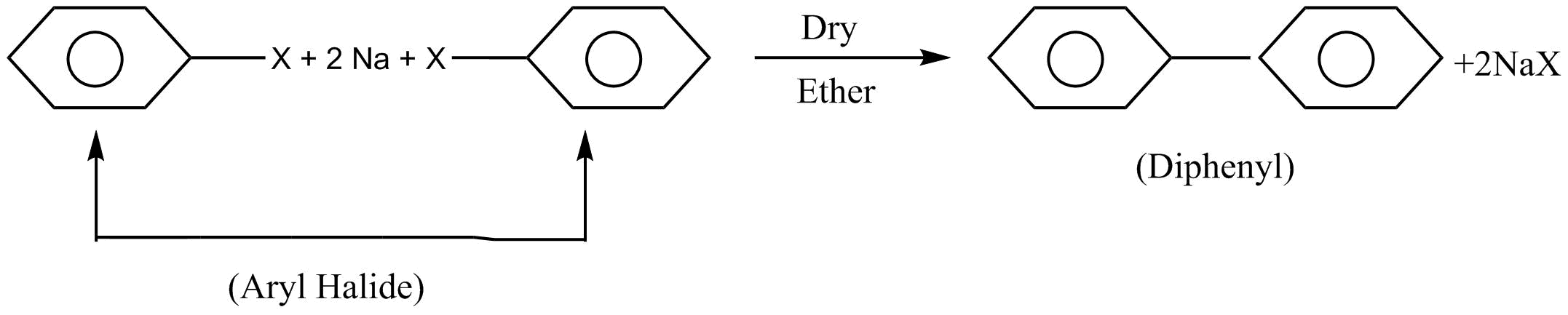
Let us firstly discuss all the reactions given. First of all is a fitting reaction. When $2$ moles of aryl halide are reacted with sodium $\left( {Na} \right)$ metal in presence of dry ether which leads to the formation of diphenyl. This reaction is known as a fitting reaction.
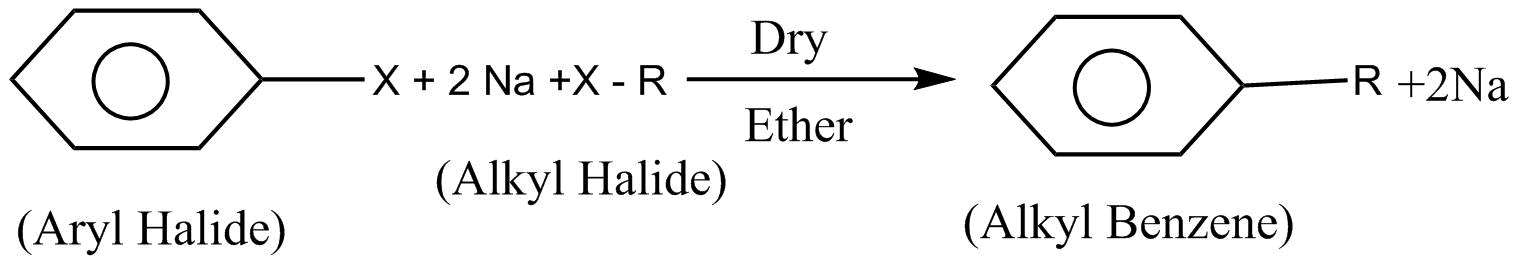
The next reaction given is a Wurtz fittig reaction. In this reaction alkyl halide is reacted with aryl halide and sodium $\left( {Na} \right)$metal in presence of dry ether which leads to the formation of alkyl benzene.
The wurtz reaction:- In this reaction, $2$ moles of alkyl halide is reacted with sodium $\left( {Na} \right)$ metal in presence of dry ether which leads to formation of alkane.
${R_1} - X + 2Na + X - {R_2}\xrightarrow{{{\text{dry ether}}}}{R_1} - {R_2} + 2NaX$
The next type of given reaction is cannizzaro reaction. It is given by those carbonyl compounds in which $\alpha - $hydrogen is absent. It is a disproportionation reds reaction
In the Benzaldehyde, $\alpha $-hydrogen is absent. so the $2$ moles of Benzaldehyde undergo cannizzaro reaction to given benzyl alcohol
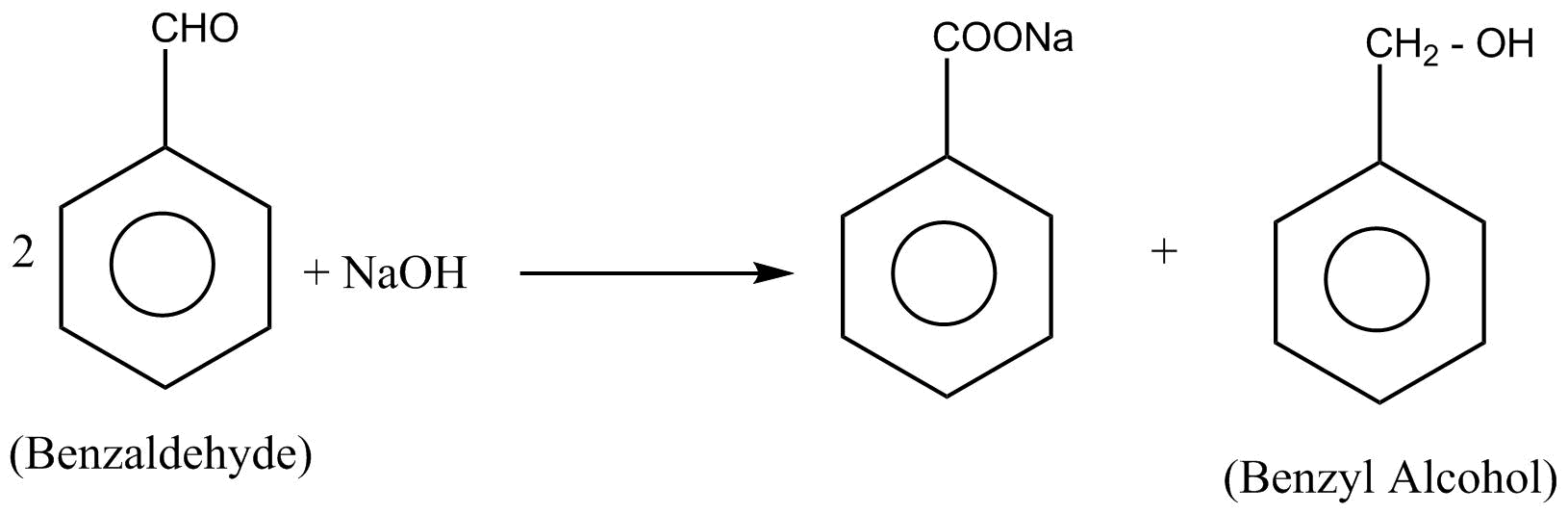
Hence conversion of Benzaldehyde into benzyl alcohol takes place by cannizzaro reaction
Hence option (D) is the correct answer.
Note:
Cannizzaro reaction can be done also with $KOH$. The base used here should be Concentrated. The Cannizzaro process is a redox reaction involving transfer of a hydride from one substrate molecule to the other.
Complete step by step answer:
Let us firstly discuss all the reactions given. First of all is a fitting reaction. When $2$ moles of aryl halide are reacted with sodium $\left( {Na} \right)$ metal in presence of dry ether which leads to the formation of diphenyl. This reaction is known as a fitting reaction.

The next reaction given is a Wurtz fittig reaction. In this reaction alkyl halide is reacted with aryl halide and sodium $\left( {Na} \right)$metal in presence of dry ether which leads to the formation of alkyl benzene.

The wurtz reaction:- In this reaction, $2$ moles of alkyl halide is reacted with sodium $\left( {Na} \right)$ metal in presence of dry ether which leads to formation of alkane.
${R_1} - X + 2Na + X - {R_2}\xrightarrow{{{\text{dry ether}}}}{R_1} - {R_2} + 2NaX$
The next type of given reaction is cannizzaro reaction. It is given by those carbonyl compounds in which $\alpha - $hydrogen is absent. It is a disproportionation reds reaction
In the Benzaldehyde, $\alpha $-hydrogen is absent. so the $2$ moles of Benzaldehyde undergo cannizzaro reaction to given benzyl alcohol

Hence conversion of Benzaldehyde into benzyl alcohol takes place by cannizzaro reaction
Hence option (D) is the correct answer.
Note:
Cannizzaro reaction can be done also with $KOH$. The base used here should be Concentrated. The Cannizzaro process is a redox reaction involving transfer of a hydride from one substrate molecule to the other.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




