
The –COOH group in benzene ring is:
(A) ortho directly
(B) para directly
(C) ortho and para directly
(D) meta directly
Answer
570.6k+ views
Hint:In electrophilic aromatic substitution, a substituent that favours electrophilic attack meta to the substituent. Aromatic compounds react by electrophilic aromatic substitution reactions in which the aromaticity of the ring system is preserved.
Complete step by step answer:
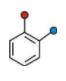
This is Ortho Directing Benzene Ring
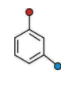
This is Meta Directing Benzene Ring
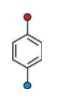
This is Para Directing Benzene Ring
Benzene is an organic chemical compound with the molecular formula \[{C_6}{H_6}\]. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. As it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon.
Benzene is a natural constituent of crude oil and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon, the second annulene. It is sometimes abbreviated PhH. Benzene is a colourless and highly flammable liquid with a sweet smell, and is responsible for the aroma around petrol stations, such as ethyl benzene and cumene, of which billions of kilograms are produced annually. As benzene has a high octane number, aromatic derivatives like toluene and xylene typically comprise upto 25% of gasoline. Benzene itself has been limited to less than 1% in gasoline because it is a known human carcinogen. Most non-industrial applications have been limited as well for the same reason.
If substituent attached on benzene ring is electron withdrawing i.e. \[{\rm{C}}{{\rm{O}}_{\rm{2}}}{\rm{R}},{\rm{ }} - {\rm{N}}{{\rm{O}}_2},\;{\rm{ - COOH}}\] then it is meta directing except halogens (-X) which are deactivating in nature but direct ortho / para. If substituent attached on the benzene ring is electron donating i.e. \[ - {\rm{OH}},{\rm{N}}{{\rm{H}}_{\rm{2}}}\;{\rm{or}}\; - {\rm{NR}},\; - {\rm{OR}}\] etc then these are ortho and para directing.
\[{\rm{ - COOH}}\] group is an electron withdrawing group which deactivates the benzene ring towards the electrophilic substitution reaction. So, the electron deficient electrophile always attacks at the meta position. Therefore, \[{\rm{ - COOH}}\] is meta directing in electrophilic aromatic substitution reactions.
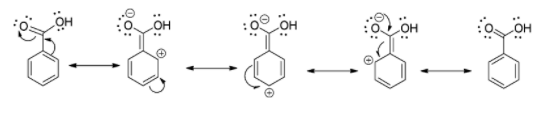
The correct answer will be (D) meta directly.
Note:
In this type of questions where the categories of benzene ring the meta-product dominates, and the ortho- and para- products are minor. Similar example: Nitration of trifluoromethyl benzene gives the meta product in about 90% yield.
Complete step by step answer:

This is Ortho Directing Benzene Ring

This is Meta Directing Benzene Ring

This is Para Directing Benzene Ring
Benzene is an organic chemical compound with the molecular formula \[{C_6}{H_6}\]. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. As it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon.
Benzene is a natural constituent of crude oil and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon, the second annulene. It is sometimes abbreviated PhH. Benzene is a colourless and highly flammable liquid with a sweet smell, and is responsible for the aroma around petrol stations, such as ethyl benzene and cumene, of which billions of kilograms are produced annually. As benzene has a high octane number, aromatic derivatives like toluene and xylene typically comprise upto 25% of gasoline. Benzene itself has been limited to less than 1% in gasoline because it is a known human carcinogen. Most non-industrial applications have been limited as well for the same reason.
If substituent attached on benzene ring is electron withdrawing i.e. \[{\rm{C}}{{\rm{O}}_{\rm{2}}}{\rm{R}},{\rm{ }} - {\rm{N}}{{\rm{O}}_2},\;{\rm{ - COOH}}\] then it is meta directing except halogens (-X) which are deactivating in nature but direct ortho / para. If substituent attached on the benzene ring is electron donating i.e. \[ - {\rm{OH}},{\rm{N}}{{\rm{H}}_{\rm{2}}}\;{\rm{or}}\; - {\rm{NR}},\; - {\rm{OR}}\] etc then these are ortho and para directing.
\[{\rm{ - COOH}}\] group is an electron withdrawing group which deactivates the benzene ring towards the electrophilic substitution reaction. So, the electron deficient electrophile always attacks at the meta position. Therefore, \[{\rm{ - COOH}}\] is meta directing in electrophilic aromatic substitution reactions.

The correct answer will be (D) meta directly.
Note:
In this type of questions where the categories of benzene ring the meta-product dominates, and the ortho- and para- products are minor. Similar example: Nitration of trifluoromethyl benzene gives the meta product in about 90% yield.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




