
The coordination number of calcium fluoride type structure is (cation : anion):
(a) 1:2
(b) 4:4
(c) 4:8
(d) 8:4
Answer
573.6k+ views
Hint: Calcium fluoride has a fluorite type of structure in which the cation forms the FCC unit cell while the anions occupy the tetrahedral sites.
Complete step by step solution:
For solving this question, we need to understand the structure of the face centred cubic unit cell. The diagram of Face centred cubic unit cell is given below:
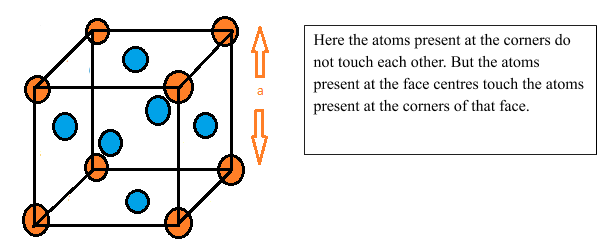
Here atoms are present at the corners as well as at the face centres. The atoms present at the corners are shared among 8 unit cells while the atoms present at face centres are shared among 2 unit cells. Therefore the total number of atoms per unit cell is = $8\times \cfrac { 1 }{ 8 } +6\times \cfrac { 1 }{ 2 } =4$
Let us calculate the packing efficiency for this unit cell. For that we need to establish a relationship between r and a where r is the radius of the atoms and a is the side of the cube.
Since the atoms present at the face centers are touching the atoms present at the corners of that face, therefore:
$a=\sqrt { 8 } r$.
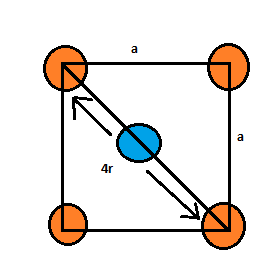
According to the Pythagoras equation:
$(4r{ ) }^{ 2 }={ a }^{ 2 }+{ a }^{ 2 }$
Therefore, $a=\sqrt { 8 } r$.
The volume occupied by the atoms present in the unit cell is = $4\times \dfrac { 4 }{ 3 } \pi { r }^{ 3 }$
The volume of the unit cell will be:
Since $a=\sqrt { 8 } r$, therefore
${ a }^{ 3 }=8\sqrt { 8 } { r }^{ 3 }$
The packing efficiency of the unit cell is = $\cfrac { 4\times \cfrac { 4 }{ 3 } \pi { r }^{ 3 } }{ 8\sqrt { 8 } { r }^{ 3 } } =0.74$
Hence a face centred cubic unit cell has some voids present in its structure. These voids are octahedral voids and tetrahedral voids. The octahedral voids have a coordination number of six while the tetrahedral voids have a coordination number of four. If there are N atoms per unit cell, then there will be N octahedral voids and 2N tetrahedral voids.
In ${ CaF }_{ 2 }$, the ${ Ca }^{ 2+ }$ ions occupy all the corner and the face centres positions in the face centred cubic unit cell while the ${ F }^{ - }$ ions occupy all the tetrahedral voids. Therefore the coordination number of calcium fluoride type structure will be 8:4.
Hence the correct answer is (d) 8:4
Note: $ { CaF }_{ 2 }$ has a fluorite type of structure. In a fluorite type of structure, the cations form the FCC unit cell while the anions occupy the tetrahedral sites. There are some compounds that have an anti-fluorite type of structure. In these compounds the anions form the FCC structure while the cations occupy the tetrahedral sites. For example ${ Mg }_{ 2 }Si$.
Complete step by step solution:
For solving this question, we need to understand the structure of the face centred cubic unit cell. The diagram of Face centred cubic unit cell is given below:

Here atoms are present at the corners as well as at the face centres. The atoms present at the corners are shared among 8 unit cells while the atoms present at face centres are shared among 2 unit cells. Therefore the total number of atoms per unit cell is = $8\times \cfrac { 1 }{ 8 } +6\times \cfrac { 1 }{ 2 } =4$
Let us calculate the packing efficiency for this unit cell. For that we need to establish a relationship between r and a where r is the radius of the atoms and a is the side of the cube.
Since the atoms present at the face centers are touching the atoms present at the corners of that face, therefore:
$a=\sqrt { 8 } r$.

According to the Pythagoras equation:
$(4r{ ) }^{ 2 }={ a }^{ 2 }+{ a }^{ 2 }$
Therefore, $a=\sqrt { 8 } r$.
The volume occupied by the atoms present in the unit cell is = $4\times \dfrac { 4 }{ 3 } \pi { r }^{ 3 }$
The volume of the unit cell will be:
Since $a=\sqrt { 8 } r$, therefore
${ a }^{ 3 }=8\sqrt { 8 } { r }^{ 3 }$
The packing efficiency of the unit cell is = $\cfrac { 4\times \cfrac { 4 }{ 3 } \pi { r }^{ 3 } }{ 8\sqrt { 8 } { r }^{ 3 } } =0.74$
Hence a face centred cubic unit cell has some voids present in its structure. These voids are octahedral voids and tetrahedral voids. The octahedral voids have a coordination number of six while the tetrahedral voids have a coordination number of four. If there are N atoms per unit cell, then there will be N octahedral voids and 2N tetrahedral voids.
In ${ CaF }_{ 2 }$, the ${ Ca }^{ 2+ }$ ions occupy all the corner and the face centres positions in the face centred cubic unit cell while the ${ F }^{ - }$ ions occupy all the tetrahedral voids. Therefore the coordination number of calcium fluoride type structure will be 8:4.
Hence the correct answer is (d) 8:4
Note: $ { CaF }_{ 2 }$ has a fluorite type of structure. In a fluorite type of structure, the cations form the FCC unit cell while the anions occupy the tetrahedral sites. There are some compounds that have an anti-fluorite type of structure. In these compounds the anions form the FCC structure while the cations occupy the tetrahedral sites. For example ${ Mg }_{ 2 }Si$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




