
The correct combination of names for isomeric alcohols with molecular formula ${C_4}{H_{10}}O$ is/are :
a.) tert-butanol and 2-methylpropan-2-ol
b.) tert-butanol and 1,1-dimethyl ethan-1-ol
c.) n-butanol and butan-1-ol
d.) isobutyl alcohol and 2-methylpropan-1-ol
Answer
579.3k+ views
Hint: The isomers are those species that contain the same molecular formula. We need to draw the structures of all the compounds given to us in the question and then calculate the number of carbon, hydrogen and oxygen atoms present in the structures. After this, we can derive their molecular formula and thus check whether it is similar to the given in question.
Complete step by step answer:
The alcohol is one that contains OH groups. The isomers are the molecules that have the same molecular formula but different arrangement of atoms in space.
Thus, we have to see the molecules given in options. The option of having both the molecules with ${C_4}{H_{10}}O$ molecular formula will be the correct answer.
So, let us see the options one by one.
The first option contains the molecules tert-butanol and 2-methylpropan-2-ol. The structures of these molecules are as given below.
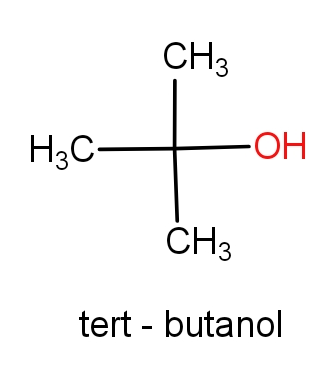
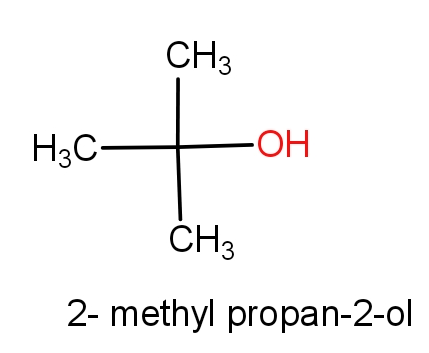
Both the molecules have the same structures but names are different. These both the molecules have ${C_4}{H_{10}}O$ molecular formula. So, this can be the correct answer.
The second option is tert-butanol and 1,1-dimethyl ethan-1-ol. The structures of these molecules are as given below.
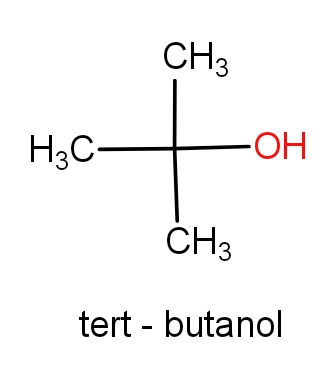
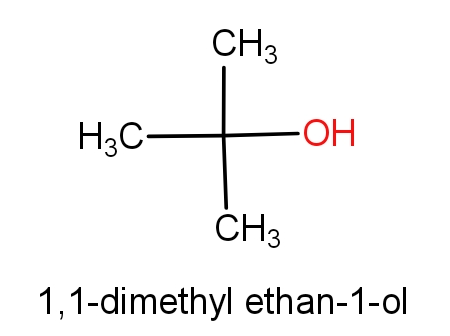
Both the molecules have the same structures but names are different. These both the molecules have ${C_4}{H_{10}}O$ molecular formula. So, this can be the correct answer.
The third option given contains n-butanol and butan-1-ol. The structures of these molecules are as given below.
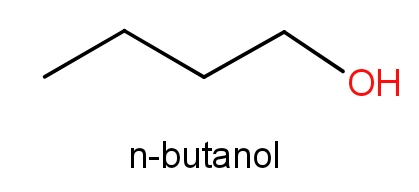
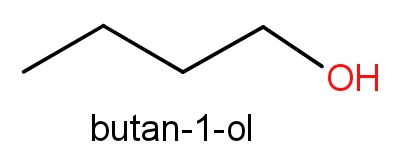
Both these molecules even have the same structures but names are different. These both the molecules have ${C_4}{H_{10}}O$ molecular formula. So, even this can be the correct answer.
The fourth option has isobutyl alcohol and 2-methylpropan-1-ol.
The structures of these molecules are as given below.
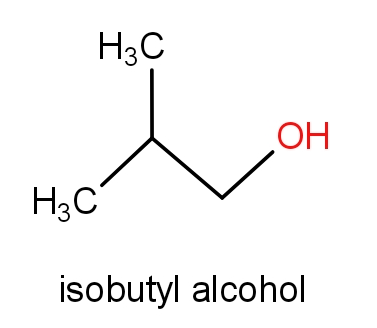
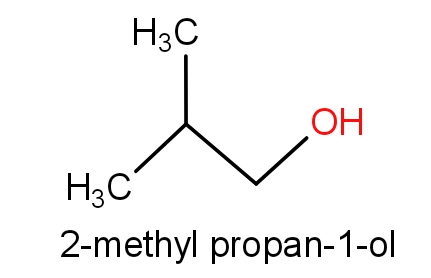
Both the molecules consist of the same structures but names are different. These both the molecules have ${C_4}{H_{10}}O$ molecular formula. So, this can also be the correct answer.
So, the correct answer is “Option A, B, C and D”.
Note: For drawing the structures from names, one should know about the IUPAC nomenclature of compounds. The word ‘n’ before name is equal to first position only. Further, the word tert means tertiary. Some of the molecules above have IUPAC names while some have common names. So, we should know both the types of names.
Complete step by step answer:
The alcohol is one that contains OH groups. The isomers are the molecules that have the same molecular formula but different arrangement of atoms in space.
Thus, we have to see the molecules given in options. The option of having both the molecules with ${C_4}{H_{10}}O$ molecular formula will be the correct answer.
So, let us see the options one by one.
The first option contains the molecules tert-butanol and 2-methylpropan-2-ol. The structures of these molecules are as given below.


Both the molecules have the same structures but names are different. These both the molecules have ${C_4}{H_{10}}O$ molecular formula. So, this can be the correct answer.
The second option is tert-butanol and 1,1-dimethyl ethan-1-ol. The structures of these molecules are as given below.


Both the molecules have the same structures but names are different. These both the molecules have ${C_4}{H_{10}}O$ molecular formula. So, this can be the correct answer.
The third option given contains n-butanol and butan-1-ol. The structures of these molecules are as given below.


Both these molecules even have the same structures but names are different. These both the molecules have ${C_4}{H_{10}}O$ molecular formula. So, even this can be the correct answer.
The fourth option has isobutyl alcohol and 2-methylpropan-1-ol.
The structures of these molecules are as given below.


Both the molecules consist of the same structures but names are different. These both the molecules have ${C_4}{H_{10}}O$ molecular formula. So, this can also be the correct answer.
So, the correct answer is “Option A, B, C and D”.
Note: For drawing the structures from names, one should know about the IUPAC nomenclature of compounds. The word ‘n’ before name is equal to first position only. Further, the word tert means tertiary. Some of the molecules above have IUPAC names while some have common names. So, we should know both the types of names.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




