
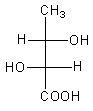
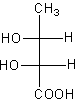
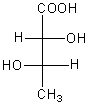
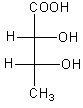
The correct fischer projection formula of $2$R, $3$S$ - 2,3 - $ dihydroxy butanoic acid is:
(A)
(B)
(C)
(D)




Answer
558.6k+ views
Hint:We should know to write the nomenclature of alkane and RS nomenclature. We will determine the chiral centre. Then we will give priority to all groups present around the chiral centre and check the direction of rotation to decide the nomenclature of the chiral centre.
Complete step-by-step answer:For a compound to be optically active, the presence of a chiral centre is necessary. A carbon whose all valancy are satisfied by different substituents is known as a chiral centre. The compound having no chiral centre is known as optical inactive.To determine the nomenclature, we will select the longest carbon chain.Then we will give the numbering to the chain as that all substituents will get lowest numbering.Then we will arrange all substituents in alphabetical order to write the name.After writing the IUPAC name we will we will decide the priority of group around the chiral centre then move from group having priority one to group having priority three as $1 - 2 - 3$. If the direction if clockwise the name of stereocenter will be R and if the direction of rotation is anticlockwise the name of stereo centre will be S. The group having atomic number will have high priority.The given IUPAC name of compound is of $2$R, $3$S$ - 2,3 - $ dihydroxy butanoic acid.The longest chain is of four carbon atoms. Which has two hydroxyl groups at second and third carbon atoms.
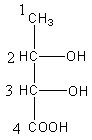
All options represent the correct structure of the compound now we have to determine the stereo.
We will check the stereo of all the compounds given in all options to determine the correct option.
In option A, Carbon-second and third are chiral centres as both have four different substituents. At carbon-, -OH group has priority-$1$, whole structure $ - \,{\text{CH(OH)COOH}}$ has priority-$2$ and $ - {\text{C}}{{\text{H}}_3}$ has priority-$3$. The direction of rotation is clockwise, so the name of the stereocenter is ${\text{2(R)}}$. At carbon-$3$, -OH group has priority-$1$, group $ - \,{\text{COOH}}$ has priority-$2$ and whole structure $ - \,{\text{CH(OH)C}}{{\text{H}}_3}$ has priority-$3$. The direction of rotation is anticlockwise so, the name of the stereocenter is ${\text{3(S)}}$.
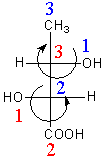
The IUPAC name of compound A is $2$R, $3$S$ - 2,3 - $dihydroxy butanoic acid.
So, the correct fischer projection formula of $2$R, $3$S$ - 2,3 - $dihydroxy butanoic acid is,
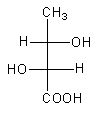
Therefore, option (C) is correct.
Note:The molecules that are mirror images of each other but are non-superimposable are known as enantiomers. The relation between naming of enantiomers is, RS-SR, SR-RS, RR-SS, SS-RR. Means if one compound has naming RS and it’s another image has naming SR then both are enantiomers. The chiral molecule rotates the light in a clockwise direction are known as dextrorotatory and represented by \[\left( + \right)\] sign and the chiral molecule rotate the light in an anticlockwise direction are known as laevorotatory and represented by \[\left( - \right)\] sign.
Complete step-by-step answer:For a compound to be optically active, the presence of a chiral centre is necessary. A carbon whose all valancy are satisfied by different substituents is known as a chiral centre. The compound having no chiral centre is known as optical inactive.To determine the nomenclature, we will select the longest carbon chain.Then we will give the numbering to the chain as that all substituents will get lowest numbering.Then we will arrange all substituents in alphabetical order to write the name.After writing the IUPAC name we will we will decide the priority of group around the chiral centre then move from group having priority one to group having priority three as $1 - 2 - 3$. If the direction if clockwise the name of stereocenter will be R and if the direction of rotation is anticlockwise the name of stereo centre will be S. The group having atomic number will have high priority.The given IUPAC name of compound is of $2$R, $3$S$ - 2,3 - $ dihydroxy butanoic acid.The longest chain is of four carbon atoms. Which has two hydroxyl groups at second and third carbon atoms.

All options represent the correct structure of the compound now we have to determine the stereo.
We will check the stereo of all the compounds given in all options to determine the correct option.
In option A, Carbon-second and third are chiral centres as both have four different substituents. At carbon-, -OH group has priority-$1$, whole structure $ - \,{\text{CH(OH)COOH}}$ has priority-$2$ and $ - {\text{C}}{{\text{H}}_3}$ has priority-$3$. The direction of rotation is clockwise, so the name of the stereocenter is ${\text{2(R)}}$. At carbon-$3$, -OH group has priority-$1$, group $ - \,{\text{COOH}}$ has priority-$2$ and whole structure $ - \,{\text{CH(OH)C}}{{\text{H}}_3}$ has priority-$3$. The direction of rotation is anticlockwise so, the name of the stereocenter is ${\text{3(S)}}$.

The IUPAC name of compound A is $2$R, $3$S$ - 2,3 - $dihydroxy butanoic acid.
So, the correct fischer projection formula of $2$R, $3$S$ - 2,3 - $dihydroxy butanoic acid is,

Therefore, option (C) is correct.
Note:The molecules that are mirror images of each other but are non-superimposable are known as enantiomers. The relation between naming of enantiomers is, RS-SR, SR-RS, RR-SS, SS-RR. Means if one compound has naming RS and it’s another image has naming SR then both are enantiomers. The chiral molecule rotates the light in a clockwise direction are known as dextrorotatory and represented by \[\left( + \right)\] sign and the chiral molecule rotate the light in an anticlockwise direction are known as laevorotatory and represented by \[\left( - \right)\] sign.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




