
The correct IUPAC name of the ether \[2\]-methylpropoxy methane is:
A. \[1\]-Methoxy-\[2\]-methyl butane
B. \[1\]-Methoxy-\[2\]-methyl propane
C. \[2\]-Methoxy-\[1\]-methyl propane
D. \[2\]-Methoxy-\[1\]-methyl butane
Answer
585.6k+ views
Hint: The IUPAC refers to International Union of Pure and Applied Chemistry. This gives a method for naming the organic compounds. Ether is the class of organic compounds where the two alkyl groups are attached by an oxygen atom which can be represented as.
-$R-O-R’$
Complete step by step answer: In order to find the IUPAC name of the given compound we have to know the rules of IUPAC for naming the ethers.
In common practice different alkyl/aryl groups attached to the oxygen atom on either side are named in alphabetical order and adding the word ether to it at the end.
In case the same group is attached to the oxygen atom on either side are named with adding prefixes such as “di”. Thus the nomenclature of ethers is done by adding “di” before the alkyl/aryl groups attached to the oxygen atom.
The IUPAC nomenclature of ethers is followed using different guidelines. When a substituent group containing more number of carbon atoms is present as the alkyl/aryl group, it is chosen as parent hydrocarbon. The substituent group which is attached to the other side of oxygen atom is named with a prefix “oxy”. For example, the compound \[C{H_3}O{C_2}{H_5}\] has the name \[1\]-methoxy ethane.
For determining the parent hydrocarbon and for the numbering of the carbon atoms let us draw the structure of the ether \[2\]-methylpropoxy methane.
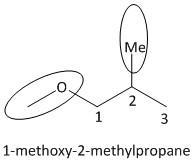
From the structure of the compound, the parent hydrocarbon attached to the oxygen atom is clearly propane. The other substituent attached to oxygen is methyl. Thus the numbering of carbon atoms will start from the carbon which is attached to oxygen atom (as atomic number of oxygen is higher than carbon in periodic table). Another methyl group is attached to the \[C - 2\] carbon of the propane chain. Thus the correct IUPAC name of the ether \[2\]-methylpropoxy methane is \[1\]-methoxy-\[2\]-methylpropane i.e. option B is the correct answer.
Note: In case if an aryl group like phenyl group is attached to a side of oxygen atom like in \[C{H_3}O{C_6}{H_5}\] or\[C{H_3}OC{H_2}{C_6}{H_5}\]. The IUPAC name of the compounds will be anisole for the first compound and (methoxymethyl)benzene for the second.
-$R-O-R’$
Complete step by step answer: In order to find the IUPAC name of the given compound we have to know the rules of IUPAC for naming the ethers.
In common practice different alkyl/aryl groups attached to the oxygen atom on either side are named in alphabetical order and adding the word ether to it at the end.
In case the same group is attached to the oxygen atom on either side are named with adding prefixes such as “di”. Thus the nomenclature of ethers is done by adding “di” before the alkyl/aryl groups attached to the oxygen atom.
The IUPAC nomenclature of ethers is followed using different guidelines. When a substituent group containing more number of carbon atoms is present as the alkyl/aryl group, it is chosen as parent hydrocarbon. The substituent group which is attached to the other side of oxygen atom is named with a prefix “oxy”. For example, the compound \[C{H_3}O{C_2}{H_5}\] has the name \[1\]-methoxy ethane.
For determining the parent hydrocarbon and for the numbering of the carbon atoms let us draw the structure of the ether \[2\]-methylpropoxy methane.

From the structure of the compound, the parent hydrocarbon attached to the oxygen atom is clearly propane. The other substituent attached to oxygen is methyl. Thus the numbering of carbon atoms will start from the carbon which is attached to oxygen atom (as atomic number of oxygen is higher than carbon in periodic table). Another methyl group is attached to the \[C - 2\] carbon of the propane chain. Thus the correct IUPAC name of the ether \[2\]-methylpropoxy methane is \[1\]-methoxy-\[2\]-methylpropane i.e. option B is the correct answer.
Note: In case if an aryl group like phenyl group is attached to a side of oxygen atom like in \[C{H_3}O{C_6}{H_5}\] or\[C{H_3}OC{H_2}{C_6}{H_5}\]. The IUPAC name of the compounds will be anisole for the first compound and (methoxymethyl)benzene for the second.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




