
The correct name of, $\left[ {Ni\left( {DM{G_2}} \right)} \right]$a cherry red precipitate is:
(A) bis (dimethyl glyoximato) nickel (II)
(B) bis (dimethyl glyoximato) nickelate (II)
(C) bis (dimethylglyoxime) nickel (II)
(D) bis (dimethylglyoxime) nickelate (II)
Answer
587.7k+ views
Hint: While naming such complex compounds it is important to take care of the part that the name of the ligands (an ion or molecule attached to a metal atom by coordination bonding is known as ligand) should be mentioned, before the name of the metal ion with its oxidation state and ‘bis-‘ is used to describe how many times a certain ligand is attached to the central metal.
Complete step by step answer:
The chemical structure of the compound with a chemical formula of $\left[ {Ne\left( {DM{G_2}} \right)} \right]$ is shown below:
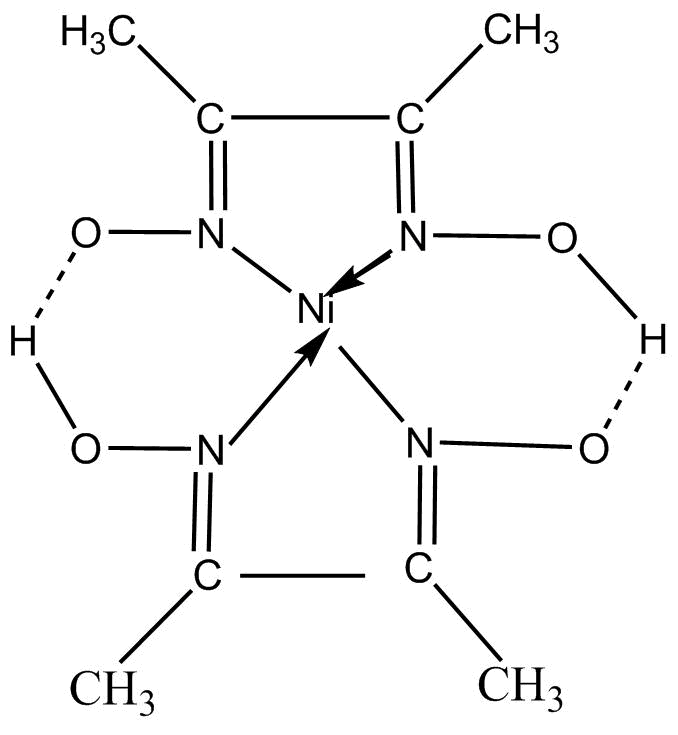
It can also be written as: $Ni\left[ {ONC\left( {C{H_3}} \right)C\left( {C{H_3}} \right)NOH} \right]$
Now, it can be clearly seen from the structure of the compound that the central metal in this compound nickel (Ni) with an oxidation state (II) and dimethylglyoxime is acting as a ligand. Since, two molecules of the same ligand that is dimethylglyoxime, are attached to the central atom Nickel, therefore the word ‘bis’ is used just to indicate the attachment of two same ligands with the central atom.
Since, ligand which is attached to the central atom is an oxygen containing group, thus the word ‘ate’ is being added to the name of the liquid. So, now the name of the ligand becomes – ‘dimethylglyoximato’ as there are two oxygen-containing ligands attached to Nickel.
We know that, for the naming of such complex coordinate compounds, the name of the ligand should be written before the name of the central atom. So, the name of this given compound $\left[ {Ne\left( {DM{G_2}} \right)} \right]$ is: ‘bis (dimethylglyoximato) nickel (II)’.
So the correct option is (A).
Additional Information
The compound $\left[ {Ne\left( {DM{G_2}} \right)} \right]$ is having a coordinate bond between the nickel atom and the nitrogen atoms and there is also a hydrogen bond between O – H -O bonds.
Note:
$\left[ {Ne\left( {DM{G_2}} \right)} \right]$ is a square planar complex and is obtained as a flocculant rose – red precipitate in the gravimetric determination of nickel. Also, it has high stability and low solubility due to the presence of hydrogen bonding.
Complete step by step answer:
The chemical structure of the compound with a chemical formula of $\left[ {Ne\left( {DM{G_2}} \right)} \right]$ is shown below:

It can also be written as: $Ni\left[ {ONC\left( {C{H_3}} \right)C\left( {C{H_3}} \right)NOH} \right]$
Now, it can be clearly seen from the structure of the compound that the central metal in this compound nickel (Ni) with an oxidation state (II) and dimethylglyoxime is acting as a ligand. Since, two molecules of the same ligand that is dimethylglyoxime, are attached to the central atom Nickel, therefore the word ‘bis’ is used just to indicate the attachment of two same ligands with the central atom.
Since, ligand which is attached to the central atom is an oxygen containing group, thus the word ‘ate’ is being added to the name of the liquid. So, now the name of the ligand becomes – ‘dimethylglyoximato’ as there are two oxygen-containing ligands attached to Nickel.
We know that, for the naming of such complex coordinate compounds, the name of the ligand should be written before the name of the central atom. So, the name of this given compound $\left[ {Ne\left( {DM{G_2}} \right)} \right]$ is: ‘bis (dimethylglyoximato) nickel (II)’.
So the correct option is (A).
Additional Information
The compound $\left[ {Ne\left( {DM{G_2}} \right)} \right]$ is having a coordinate bond between the nickel atom and the nitrogen atoms and there is also a hydrogen bond between O – H -O bonds.
Note:
$\left[ {Ne\left( {DM{G_2}} \right)} \right]$ is a square planar complex and is obtained as a flocculant rose – red precipitate in the gravimetric determination of nickel. Also, it has high stability and low solubility due to the presence of hydrogen bonding.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




