
The correct order of ionic radius is:
A.$Ce\, > \,Sm\, > \,Tb\, > \,Lu$
B.$Lu\, > \,Tb\, > \,Sm\, > \,Ce$
C.$Tb\, > \,Lu\, > \,Sm\, > \,Ce$
D.$Sm\, > \,Tb\, > \,Lu\, > \,Ce$
Answer
551.7k+ views
Hint:The above elements are from the Lanthanides series which are having a total of $15$ elements starting from Lanthanum and ending with lutetium. If you know the periodic trends in periodic tables then you will see that radii decreases as we move from left to right and it increases on moving down the group.
Complete step-by-step answer:In periodic table, it was seen that when arrangements of elements are seen from left to right there is a decrease in atomic radii as well as ionic radii. This decrease can be understood on the basis of size of atom and also from the nuclear attraction that electrons feel from the nucleus. If we see the lanthanides, they have elements as lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium and al last we have lutetium. Among these elements we have to see the periodic ionic radii trends among cerium, samarium, terbium and lutetium.
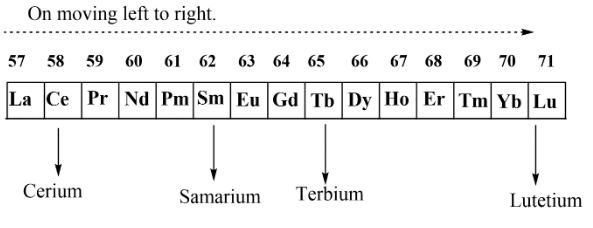
As we know that there is always a decrease in ionic radii, thus as we move from cerium to lutetium there will be a decrease in ionic radii. So by this biggest ion will be cerium then after that we have samarium, then terbium and at last we have lutetium. So the order will be written as
$Ce\, > \,Sm\, > \,Tb\, > \,Lu$
Note:Whenever we have to compare the atomic size or size of the ions we firstly have to write all the elements of groups or periods according to them see which elements you have to compare for. Like above we circled the elements that we have to compare then by seeing their trend from left to right movement. There are some exceptions too.
Complete step-by-step answer:In periodic table, it was seen that when arrangements of elements are seen from left to right there is a decrease in atomic radii as well as ionic radii. This decrease can be understood on the basis of size of atom and also from the nuclear attraction that electrons feel from the nucleus. If we see the lanthanides, they have elements as lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium and al last we have lutetium. Among these elements we have to see the periodic ionic radii trends among cerium, samarium, terbium and lutetium.

As we know that there is always a decrease in ionic radii, thus as we move from cerium to lutetium there will be a decrease in ionic radii. So by this biggest ion will be cerium then after that we have samarium, then terbium and at last we have lutetium. So the order will be written as
$Ce\, > \,Sm\, > \,Tb\, > \,Lu$
Note:Whenever we have to compare the atomic size or size of the ions we firstly have to write all the elements of groups or periods according to them see which elements you have to compare for. Like above we circled the elements that we have to compare then by seeing their trend from left to right movement. There are some exceptions too.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




