
The correct order of rate of Wurtz reaction:
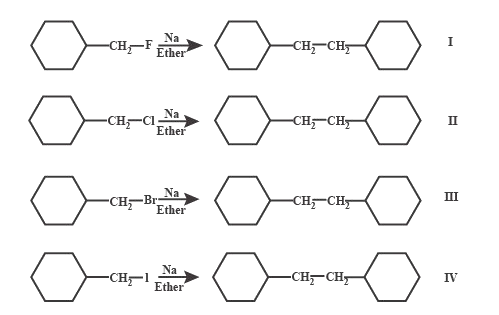
(A) $ I > II > III > IV $
(B) $ II > I > III > IV $
(C) $ IV > III > II > I $
(D) In all the rate of Wurtz reaction is same

Answer
548.4k+ views
Hint: To answer this question, you should recall the concept of Wurtz reaction. In Wurtz reaction, sodium metal reacts with two alkyl halides in the environment provided by a solution of dry ether to form a higher alkane along with a compound containing sodium and the halogen.
Complete step by step solution:
Wurtz reaction involves two different alkyl halides are coupled to yield a longer alkane chain with the use of sodium and dry ether solution. This reaction is named after the French chemist Reaction mechanism of Wurtz reaction can be represented by the equations:
$ R - X + 2Na \to {R^ - }N{a^ + } + NaX $ and $ {R^ - }N{a^ + } + R - X \to R - R + NaX $
Leaving group stability order is $ {I^ - } > B{r^ - } > C{l^ - } > {F^ - }. $
Reactivity order is dependent on the leaving group stability. If the leaving group is stable, reactivity will be more.
So, the reactivity order is $ IV > III > II > I $ .
Hence, the correct answer for this question is option C.
Note:
Wurtz Fittig reaction involves $ S{N^2} $ mechanism. This reaction proceeds through a backside attack by the nucleophile on the substrate. The nucleophile approaches the given substrate at an angle of $ {180^o} $ to the carbon-leaving group bond. Now, the leaving group is pushed out of the transition state on the opposite side of the carbon-nucleophile bond, forming the required product. It is important to note that the product is formed with an inversion of the tetrahedral geometry at the atom in the centre. You should remember the important points regarding $ S{N^2} $ reactions:
$ S{N^2} $ reactions are bimolecular reactions in which there are simultaneous bond-making and bond-breaking steps.
$ S{N^2} $ reactions do not proceed via an intermediate.
$ S{N^2} $ reactions result in inverted stereochemistry at the reaction centre.
Steric effects are particularly important in $ S{N^2} $ reactions.
Unhindered back of the substrate makes the formation of carbon-nucleophile bonds easy. Therefore, methyl and primary substrates undergo nucleophilic substitution easily.
Complete step by step solution:
Wurtz reaction involves two different alkyl halides are coupled to yield a longer alkane chain with the use of sodium and dry ether solution. This reaction is named after the French chemist Reaction mechanism of Wurtz reaction can be represented by the equations:
$ R - X + 2Na \to {R^ - }N{a^ + } + NaX $ and $ {R^ - }N{a^ + } + R - X \to R - R + NaX $
Leaving group stability order is $ {I^ - } > B{r^ - } > C{l^ - } > {F^ - }. $
Reactivity order is dependent on the leaving group stability. If the leaving group is stable, reactivity will be more.
So, the reactivity order is $ IV > III > II > I $ .
Hence, the correct answer for this question is option C.
Note:
Wurtz Fittig reaction involves $ S{N^2} $ mechanism. This reaction proceeds through a backside attack by the nucleophile on the substrate. The nucleophile approaches the given substrate at an angle of $ {180^o} $ to the carbon-leaving group bond. Now, the leaving group is pushed out of the transition state on the opposite side of the carbon-nucleophile bond, forming the required product. It is important to note that the product is formed with an inversion of the tetrahedral geometry at the atom in the centre. You should remember the important points regarding $ S{N^2} $ reactions:
$ S{N^2} $ reactions are bimolecular reactions in which there are simultaneous bond-making and bond-breaking steps.
$ S{N^2} $ reactions do not proceed via an intermediate.
$ S{N^2} $ reactions result in inverted stereochemistry at the reaction centre.
Steric effects are particularly important in $ S{N^2} $ reactions.
Unhindered back of the substrate makes the formation of carbon-nucleophile bonds easy. Therefore, methyl and primary substrates undergo nucleophilic substitution easily.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




