
The correct order of stability of given carbocations is:
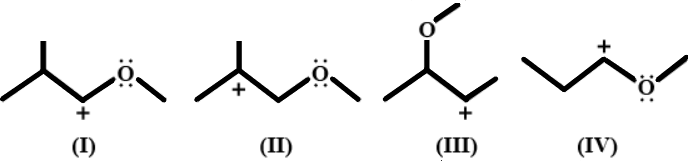
A. $I > IV > III > II$
B. $I > IV > II > III$
C. $IV > I > III > II$
D. $IV > I > II > III$

Answer
570.6k+ views
Hint: The stability of carbocations are decided by three effects: resonance, hyperconjugative and inductive effects. Their order of contribution to stability is also in that order. That is, resonance effect plays a bigger role than hyperconjugation effect, which in turn plays a bigger role than the inductive effect.
Complete step by step answer:
Resonance effect is the ability of pi electrons or lone pair in an atom to interact with a neighbouring charged particle to produce delocalisation of electrons. Resonance tends to stabilize carbocations the most as it reduces the effective positive charge on the carbocations through delocalisation of electrons. Therefore, atoms having pi electrons or lone pairs lying adjacent to carbocations will make the carbocation extremely stable, as the lone pairs can go into resonance.
As we can see from the four figures, compounds $I$ and $IV$ have oxygen atoms having two lone pairs lying adjacent to the carbocations. So, these two compounds will be more stable than $II$ and $III$, as the lone pairs can go into resonance.
Between $I$ and $IV$, $I$ will be more stable as it has an extra methyl group attached to the adjacent carbon atom. This is due to the inductive effect, which says that electron donating groups, like methyl groups tend to stabilize carbocations by pushing the cloud of electrons closer towards the positive charge, thereby reducing the effect of positive charge.
Hence, $I$ is the most stable and $IV$ is the second most stable.
Between $II$ and $III$, $II$ is more stable as it is a tertiary carbocation while $III$ has a secondary carbocation. Tertiary carbocations are more stable than secondary carbocations due to the hyperconjugation effect, which is the interaction of neighbouring sigma bonds with the empty p orbital of the carbocation, leading to stabilized structures. The number of stable, equivalent structures are dependent on the number of sigma bonds that surround the carbocation. The more the number of adjacent sigma bonds, more structures will be formed and hence, more stable is the compound. In the tertiary carbocation in compound $II$, there are eight $C - H$ sigma bonds around the carbocation, leading to eight equally stable structures, while there are only four $C - H$ sigma bonds around the carbocation in compound $III$, thus making $II$ more stable.
Hence, from our observations, correct order of stability is: $I > IV > II > III$
So, the correct answer is Option B.
Note: The stability of all carbocations is attained through minimising the effect of the positive charge. Although resonance effect plays the major role, strong electron withdrawing groups lying adjacent to the carbocation can also have a big role to play in stability, as they would increase the already positive charge on carbon by pulling away the rest of the electrons. Note that delocalisation and hyperconjugation structures don’t actually produce different structures, but the electron cloud gets uniformly distributed along the entire molecule to form a single stable structure.
Complete step by step answer:
Resonance effect is the ability of pi electrons or lone pair in an atom to interact with a neighbouring charged particle to produce delocalisation of electrons. Resonance tends to stabilize carbocations the most as it reduces the effective positive charge on the carbocations through delocalisation of electrons. Therefore, atoms having pi electrons or lone pairs lying adjacent to carbocations will make the carbocation extremely stable, as the lone pairs can go into resonance.
As we can see from the four figures, compounds $I$ and $IV$ have oxygen atoms having two lone pairs lying adjacent to the carbocations. So, these two compounds will be more stable than $II$ and $III$, as the lone pairs can go into resonance.
Between $I$ and $IV$, $I$ will be more stable as it has an extra methyl group attached to the adjacent carbon atom. This is due to the inductive effect, which says that electron donating groups, like methyl groups tend to stabilize carbocations by pushing the cloud of electrons closer towards the positive charge, thereby reducing the effect of positive charge.
Hence, $I$ is the most stable and $IV$ is the second most stable.
Between $II$ and $III$, $II$ is more stable as it is a tertiary carbocation while $III$ has a secondary carbocation. Tertiary carbocations are more stable than secondary carbocations due to the hyperconjugation effect, which is the interaction of neighbouring sigma bonds with the empty p orbital of the carbocation, leading to stabilized structures. The number of stable, equivalent structures are dependent on the number of sigma bonds that surround the carbocation. The more the number of adjacent sigma bonds, more structures will be formed and hence, more stable is the compound. In the tertiary carbocation in compound $II$, there are eight $C - H$ sigma bonds around the carbocation, leading to eight equally stable structures, while there are only four $C - H$ sigma bonds around the carbocation in compound $III$, thus making $II$ more stable.
Hence, from our observations, correct order of stability is: $I > IV > II > III$
So, the correct answer is Option B.
Note: The stability of all carbocations is attained through minimising the effect of the positive charge. Although resonance effect plays the major role, strong electron withdrawing groups lying adjacent to the carbocation can also have a big role to play in stability, as they would increase the already positive charge on carbon by pulling away the rest of the electrons. Note that delocalisation and hyperconjugation structures don’t actually produce different structures, but the electron cloud gets uniformly distributed along the entire molecule to form a single stable structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




