
The correct statement regarding the comparison of staggered and eclipsed conformations of ethane is:
A) The staggered conformation of ethane is less stable than eclipsed conformation, because staggered conformation has torsional strain.
B) The eclipsed conformation of ethane is more stable than staggered conformation, because eclipsed conformation has no torsional strain.
C) The eclipsed conformation of ethane is more stable than staggered conformation even though the eclipsed conformation has torsional strain.
D) The staggered conformation of ethane is more stable than eclipsed conformation, because staggered conformation has no torsional strain.
Answer
578.1k+ views
Hint: We know that the different spatial arrangements of atoms or groups of atoms which can be converted into one another by the rotation around single bonds are known as conformations or conformational isomers. There are two types of conformations: staggered conformation and eclipsed conformation.
Complete step by step answer:
The molecular formula for ethane is \[{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\]. In the structure of ethane, two carbon atoms are bonded to each other by a single bond. Each carbon atom is bonded to three hydrogen atoms. The structure of ethane is as follows:
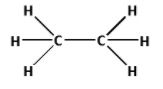
Now we can imagine that one carbon atom of the ethane undergoes rotation about the central bond while the other carbon atom is fixed. This rotation gives us an infinite number of conformations depending on the angle of rotation.
The conformations differ in spatial arrangements of atoms but the bond angles and the bond lengths remain the same.
The two conformations of ethane are: staggered conformation and eclipsed conformation.
> Staggered conformation: In staggered conformation, the hydrogen atoms of both the carbon atoms are placed widely apart. The carbon-hydrogen bond pairs of the two carbon atoms are far away from each other.
> Eclipsed conformation: In eclipsed conformation, the hydrogen atoms of both the carbon atoms are facing each other. The carbon-hydrogen bond pairs of the two carbon atoms are very close to each other.
The staggered and eclipsed conformations of ethane are as follows:
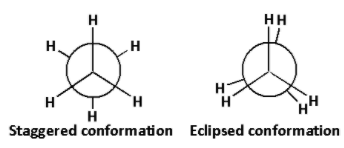
The potential energy of the molecule increases as the rotation about the central bond increases. The increase in potential energy is due to the repulsion between electrons in the bond. This increase in the potential energy is known as the torsional strain. The torsional strain thus depends on the rotation about the central bond.
From the structures of staggered and eclipsed conformations, we can conclude that the torsional strain in the staggered conformation is less than that of the eclipsed conformation.
Thus, the staggered conformation is more stable than the eclipsed conformation because staggered conformation has no torsional strain.
Thus, the correct statement regarding the comparison of staggered and eclipsed conformations of ethane is ‘the staggered conformation of ethane is more stable than eclipsed conformation, because staggered conformation has no torsional strain’.
Thus, the correct option is (D) the staggered conformation of ethane is more stable than eclipsed conformation, because staggered conformation has no torsional strain.
Note: The rotation of the carbon atom along the single bond is not free and thus, the potential energy of the molecule changes as the rotation occurs along the carbon-carbon single bond. The potential energy of the staggered conformation is minimum. The potential energy increases as the rotation increases and it is maximum for the eclipsed conformation.
Complete step by step answer:
The molecular formula for ethane is \[{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\]. In the structure of ethane, two carbon atoms are bonded to each other by a single bond. Each carbon atom is bonded to three hydrogen atoms. The structure of ethane is as follows:

Now we can imagine that one carbon atom of the ethane undergoes rotation about the central bond while the other carbon atom is fixed. This rotation gives us an infinite number of conformations depending on the angle of rotation.
The conformations differ in spatial arrangements of atoms but the bond angles and the bond lengths remain the same.
The two conformations of ethane are: staggered conformation and eclipsed conformation.
> Staggered conformation: In staggered conformation, the hydrogen atoms of both the carbon atoms are placed widely apart. The carbon-hydrogen bond pairs of the two carbon atoms are far away from each other.
> Eclipsed conformation: In eclipsed conformation, the hydrogen atoms of both the carbon atoms are facing each other. The carbon-hydrogen bond pairs of the two carbon atoms are very close to each other.
The staggered and eclipsed conformations of ethane are as follows:

The potential energy of the molecule increases as the rotation about the central bond increases. The increase in potential energy is due to the repulsion between electrons in the bond. This increase in the potential energy is known as the torsional strain. The torsional strain thus depends on the rotation about the central bond.
From the structures of staggered and eclipsed conformations, we can conclude that the torsional strain in the staggered conformation is less than that of the eclipsed conformation.
Thus, the staggered conformation is more stable than the eclipsed conformation because staggered conformation has no torsional strain.
Thus, the correct statement regarding the comparison of staggered and eclipsed conformations of ethane is ‘the staggered conformation of ethane is more stable than eclipsed conformation, because staggered conformation has no torsional strain’.
Thus, the correct option is (D) the staggered conformation of ethane is more stable than eclipsed conformation, because staggered conformation has no torsional strain.
Note: The rotation of the carbon atom along the single bond is not free and thus, the potential energy of the molecule changes as the rotation occurs along the carbon-carbon single bond. The potential energy of the staggered conformation is minimum. The potential energy increases as the rotation increases and it is maximum for the eclipsed conformation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




