
The correct statement(s) about ${O_3}$ is (are):
[This question has multiple correct options]
(A) O-O bond lengths are equal
(B) Thermal decompositions of ${O_3}$ is endothermic
(C) ${O_3}$ is diamagnetic in nature
(D) ${O_3}$ has a bent structure
Answer
579.3k+ views
Hint: ${O_3}$ is ozone and in its structure there is resonance throughout the structure. The lone pair present in the middle oxygen repels the bond pairs in it. It is unstable as compared to an oxygen molecule and so it will have higher energy than an oxygen molecule. Also any molecule will be diamagnetic only if there are no unpaired electrons present.
Complete step by step answer:
-First of all let us see what ${O_3}$ is.
It is ozone and is an allotropic molecular form of oxygen. It is basically formed when oxygen gas (${O_2}$) is passed through a high voltage potential which results in the attachment of another oxygen atom to it and forms an ozone (${O_3}$) molecule. Also ozone is unstable, pale blue coloured gas, it also has a pungent odour like that of chlorine gas and an important property of ozone is to protect the earth from the ultraviolet radiations emitted by the sun.
-Now let us take a look at the structure of ozone.
Ozone (${O_3}$) forms a bent structure because the non-bonding electrons or the lone pair takes up a lot of space and repels the bond pairs. Due to the presence of negative and positive formal charges the molecule has the ability to undergo resonance as shown below:
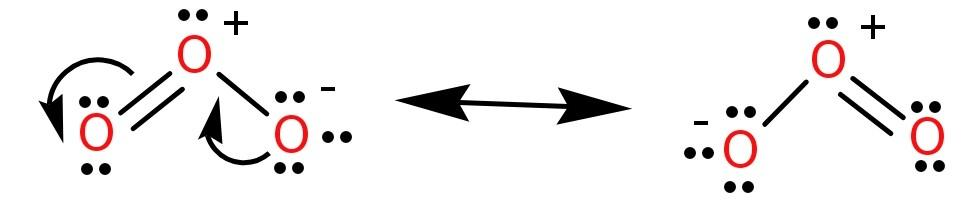
From the above resonating structures we can see that both the bonds are involved in resonance and so both the bond lengths become equal.
-We can also see that there are no unpaired electrons in ozone and hence it will be diamagnetic.
-As we discussed above ozone (${O_3}$) is an unstable form of oxygen and the oxygen molecule (${O_2}$) is quite stable. We know that a more stable molecule possesses less energy as compared to an unstable molecule and so the energy of oxygen molecules will be lesser and that of ozone will be higher. So ozone molecules being unstable will very easily decompose into oxygen molecules and since oxygen molecules possess lesser energy, the extra energy of ozone molecules will be released. This makes the process of ozone molecules an exothermic process. But the formation of ozone molecules is an endothermic process.
The involved reaction is: $2{O_3} \to 3{O_2}$; and the enthalpy here is: $\Delta {H^ \circ } = - 142kJ.mo{l^{ - 1}}$.
-Hence from the above discussion we can conclude that the correct options will be:
(A) O-O bond lengths are equal
(C) ${O_3}$ is diamagnetic in nature
(D) ${O_3}$ has a bent structure
So, the correct answer is “Option A,C and D”.
Note: Ozone has the ability to act as a powerful oxidant and hence has a large number of industrial applications. This also makes it hazardous because it can cause damage to lungs on inhalation, coughing, shortness of breath, damage to respiratory tissues, etc. Alongside of this the large ozone layer in the higher levels of atmosphere prevents us from the damaging UV radiations. But recently due to global warming this ozone layer is depleting near the arctic region, hence raising the temperature of our atmosphere.
Complete step by step answer:
-First of all let us see what ${O_3}$ is.
It is ozone and is an allotropic molecular form of oxygen. It is basically formed when oxygen gas (${O_2}$) is passed through a high voltage potential which results in the attachment of another oxygen atom to it and forms an ozone (${O_3}$) molecule. Also ozone is unstable, pale blue coloured gas, it also has a pungent odour like that of chlorine gas and an important property of ozone is to protect the earth from the ultraviolet radiations emitted by the sun.
-Now let us take a look at the structure of ozone.
Ozone (${O_3}$) forms a bent structure because the non-bonding electrons or the lone pair takes up a lot of space and repels the bond pairs. Due to the presence of negative and positive formal charges the molecule has the ability to undergo resonance as shown below:

From the above resonating structures we can see that both the bonds are involved in resonance and so both the bond lengths become equal.
-We can also see that there are no unpaired electrons in ozone and hence it will be diamagnetic.
-As we discussed above ozone (${O_3}$) is an unstable form of oxygen and the oxygen molecule (${O_2}$) is quite stable. We know that a more stable molecule possesses less energy as compared to an unstable molecule and so the energy of oxygen molecules will be lesser and that of ozone will be higher. So ozone molecules being unstable will very easily decompose into oxygen molecules and since oxygen molecules possess lesser energy, the extra energy of ozone molecules will be released. This makes the process of ozone molecules an exothermic process. But the formation of ozone molecules is an endothermic process.
The involved reaction is: $2{O_3} \to 3{O_2}$; and the enthalpy here is: $\Delta {H^ \circ } = - 142kJ.mo{l^{ - 1}}$.
-Hence from the above discussion we can conclude that the correct options will be:
(A) O-O bond lengths are equal
(C) ${O_3}$ is diamagnetic in nature
(D) ${O_3}$ has a bent structure
So, the correct answer is “Option A,C and D”.
Note: Ozone has the ability to act as a powerful oxidant and hence has a large number of industrial applications. This also makes it hazardous because it can cause damage to lungs on inhalation, coughing, shortness of breath, damage to respiratory tissues, etc. Alongside of this the large ozone layer in the higher levels of atmosphere prevents us from the damaging UV radiations. But recently due to global warming this ozone layer is depleting near the arctic region, hence raising the temperature of our atmosphere.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




