
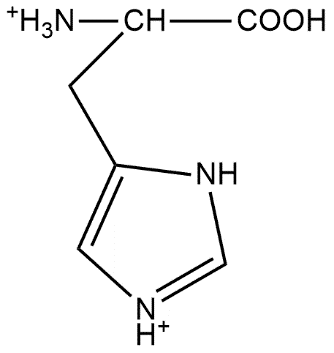
The correct structure of histidine in a strongly acidic solution (pH = 2) is:
A.
B.
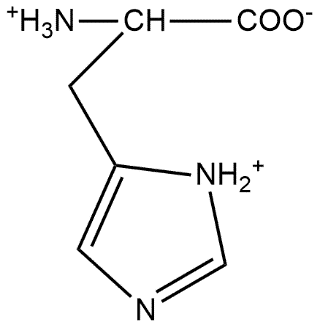
C.
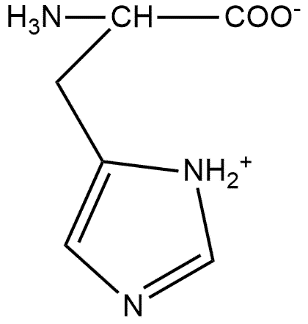
D.
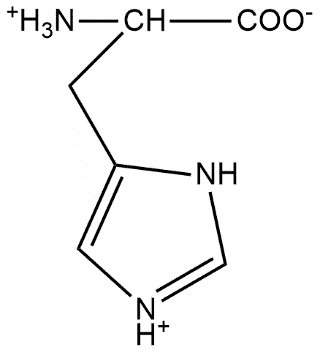




Answer
532.5k+ views
Hint: pH denotes the power of hydrogen or we can say that it represents potential of hydrogen ions. It is generally a scale which is used to represent that aqueous solution given is acidic in nature, basic in nature or in neutral form.
Complete answer:
Histidine is represented by the symbol His or H which is basically an$\alpha $ amino acid which is used in the biosynthesis of proteins. It contains an $\alpha $amino group which is in the protonated $N{{H}_{3}}^{+}$form under the biological conditions and carboxylic acid group which is in deprotonated form as shown as $CO{{O}^{-}}$under biological conditions and an imidazole side chain which is defined as partially protonated classifying it as a positively charged amino acid at physiological pH value. This type of compound is known by the name zwitterion where zwitter ion is also known by the other name called inner salt and can be defined as a molecule that contains equal number of positively and negatively charged functional groups.
On the basis of above definition we can say that the structure of histidine can be shown as:
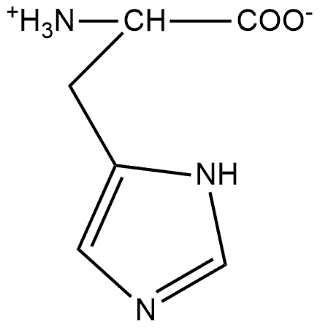
Now in strongly acidic medium $CO{{O}^{-}}$will take one of the proton and form acid and the reaction can be shown as:
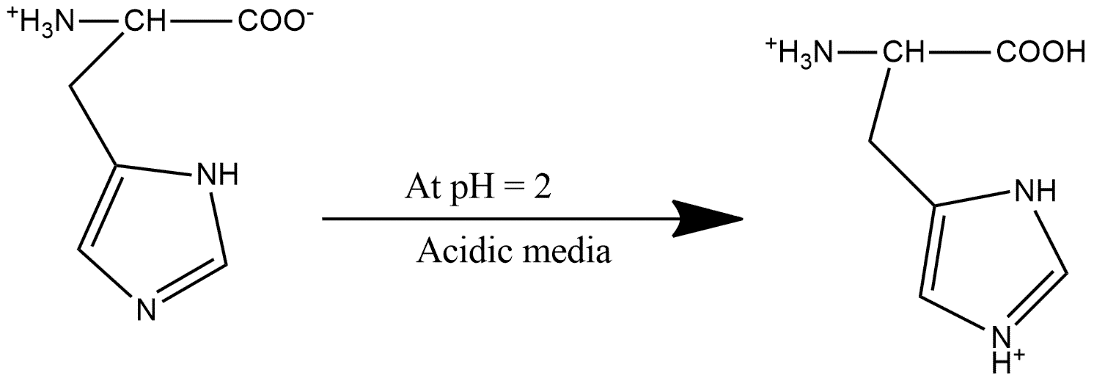
This suggests that option A is the correct answer.
Note:
Histidine was first discovered by German physicist known by the name Albrecht Kossel and Sven Gustaf Hedin in 1896. It is also a precursor to histamine i.e. a vital inflammatory agent in immune responses. The acyl radical of histidine is histidyl.
Complete answer:
Histidine is represented by the symbol His or H which is basically an$\alpha $ amino acid which is used in the biosynthesis of proteins. It contains an $\alpha $amino group which is in the protonated $N{{H}_{3}}^{+}$form under the biological conditions and carboxylic acid group which is in deprotonated form as shown as $CO{{O}^{-}}$under biological conditions and an imidazole side chain which is defined as partially protonated classifying it as a positively charged amino acid at physiological pH value. This type of compound is known by the name zwitterion where zwitter ion is also known by the other name called inner salt and can be defined as a molecule that contains equal number of positively and negatively charged functional groups.
On the basis of above definition we can say that the structure of histidine can be shown as:

Now in strongly acidic medium $CO{{O}^{-}}$will take one of the proton and form acid and the reaction can be shown as:

This suggests that option A is the correct answer.
Note:
Histidine was first discovered by German physicist known by the name Albrecht Kossel and Sven Gustaf Hedin in 1896. It is also a precursor to histamine i.e. a vital inflammatory agent in immune responses. The acyl radical of histidine is histidyl.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a labelled diagram of the human heart and label class 11 biology CBSE

What is 1s 2s 2p 3s 3p class 11 chemistry CBSE




