
The cumulated alkadiene is:
A.
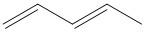
B.
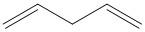
C.
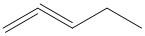
D.





Answer
561k+ views
Hint: A diene is an organic compound which contains two double bonds. The bonds are covalent in nature. A single double bond consists of a sigma and a Pi bond.
Complete step by step answer:
The alkadiene is a compound which is a hydrocarbon unit containing two double bonds. In organic chemistry the double bond containing hydrocarbons is termed as alkene.
The two double bonds present in the alkadiene are of three types. They are labeled as conjugated, non-conjugated or isolated and cumulated.
The conjugated diene are the ones in which the two double bonds are separated by a single bond. The word conjugated comes from the word conjugation. Conjugation is the movement of the two double bonded electrons to generate two resonance structures.

The isolated or non-conjugated diene are the ones in which the two double bonds are separated by more than one single bond.

The cumulated diene is the one in which the two double bonds are joined to a single carbon atom. This type of compound is termed as allene. The compounds are non-planar in nature.
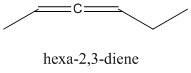
Hence option C is the correct answer, i.e. the cumulated alkadiene is
Note:
Conjugated diene are the most stable diene and are used in the preparation of polymers. Cumulated alkadiene or diene exhibits optical isomerism if the two groups attached to the terminal carbons are different. The molecule is not planar and the double bonds are perpendicular to each other.
Complete step by step answer:
The alkadiene is a compound which is a hydrocarbon unit containing two double bonds. In organic chemistry the double bond containing hydrocarbons is termed as alkene.
The two double bonds present in the alkadiene are of three types. They are labeled as conjugated, non-conjugated or isolated and cumulated.
The conjugated diene are the ones in which the two double bonds are separated by a single bond. The word conjugated comes from the word conjugation. Conjugation is the movement of the two double bonded electrons to generate two resonance structures.

The isolated or non-conjugated diene are the ones in which the two double bonds are separated by more than one single bond.

The cumulated diene is the one in which the two double bonds are joined to a single carbon atom. This type of compound is termed as allene. The compounds are non-planar in nature.
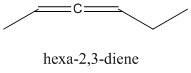
Hence option C is the correct answer, i.e. the cumulated alkadiene is

Note:
Conjugated diene are the most stable diene and are used in the preparation of polymers. Cumulated alkadiene or diene exhibits optical isomerism if the two groups attached to the terminal carbons are different. The molecule is not planar and the double bonds are perpendicular to each other.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




