
The density of $ Ca{F_2} $ (fluorite structure) is 3.18 $ gc{m^{ - 3}} $ . The length of the side of the unit cell is:
a)253 pm
b)344 pm
c)546 pm
d)273 pm
Answer
501k+ views
Hint: Crystal structure is a description of the orderly organisation of atoms, ions, or molecules in a crystalline substance used in crystallography. The inherent nature of the component particles results in symmetric patterns that recur along the major directions of three-dimensional space in matter, forming ordered structures.
Complete answer:
Calcium fluoride ( $ Ca{F_2} $ ) is an inorganic compound made up of the elements calcium and fluorine. It's an insoluble white solid. Fluorite (also known as fluorspar) is the mineral form, which is typically strongly coloured due to impurities. The fluorite structure is the crystal lattice structure of Calcium Fluoride, in which the $ C{a^{2 + }} $ ions are eight-coordinated and centred in a cube of eight $ {F^ - } $ ions. In the form of a tetrahedron, each $ {F^ - } $ is coordinated to four $ C{a^{2 + }} $
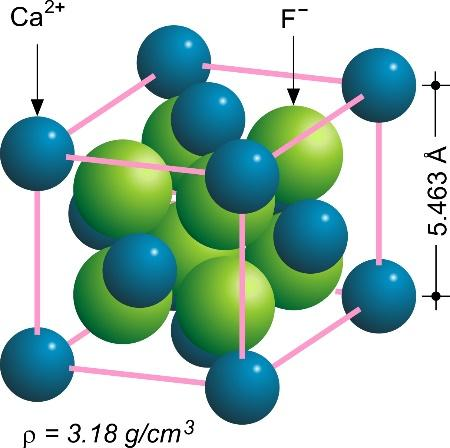
The unit cell of the structure is the smallest group of particles in the substance that makes up this repeating pattern. The symmetry and structure of the whole crystal are perfectly reflected in the unit cell, which is built up by recurrent translation of the unit cell along its major axes. The nodes of the Bravais lattice are defined by the translation vectors.
We know that
$ {\mathbf{d}} = \dfrac{{{\mathbf{zM}}}}{{{{\mathbf{N}}_{\mathbf{A}}} \times {\mathbf{V}}}} $
D = density
M= mass
$ {N_a} $ =Avogadro number
V= volume ( $ {a^3} $ )
For fluorite structure, $ {\text{z}} = 4 $ and $ {\text{M}} = 78 $
$ 3.18 = \dfrac{{4 \times 78}}{{6.023 \times {{10}^{23}} \times {a^3}}} $
$ {a^3} = \dfrac{{312}}{{6.023 \times {{10}^{23}} \times 3.18}} $
$ {a^3} = 16.28 \times {10^{ - 23}} $
$ a = 546{\text{pm}} $
Hence option c is correct.
Note:
Knowing the dimensions of the unit cell allows us to calculate the volume of the unit cell. For example, if an edge "a" has a unit cell, the volume of the unit cell may be written as " $ {a^3} $ " The density of a unit cell is defined as the ratio of its mass to its volume. The product of the number of atoms in a unit cell and the mass of each atom in a unit cell equals the mass of a unit cell.
Complete answer:
Calcium fluoride ( $ Ca{F_2} $ ) is an inorganic compound made up of the elements calcium and fluorine. It's an insoluble white solid. Fluorite (also known as fluorspar) is the mineral form, which is typically strongly coloured due to impurities. The fluorite structure is the crystal lattice structure of Calcium Fluoride, in which the $ C{a^{2 + }} $ ions are eight-coordinated and centred in a cube of eight $ {F^ - } $ ions. In the form of a tetrahedron, each $ {F^ - } $ is coordinated to four $ C{a^{2 + }} $

The unit cell of the structure is the smallest group of particles in the substance that makes up this repeating pattern. The symmetry and structure of the whole crystal are perfectly reflected in the unit cell, which is built up by recurrent translation of the unit cell along its major axes. The nodes of the Bravais lattice are defined by the translation vectors.
We know that
$ {\mathbf{d}} = \dfrac{{{\mathbf{zM}}}}{{{{\mathbf{N}}_{\mathbf{A}}} \times {\mathbf{V}}}} $
D = density
M= mass
$ {N_a} $ =Avogadro number
V= volume ( $ {a^3} $ )
For fluorite structure, $ {\text{z}} = 4 $ and $ {\text{M}} = 78 $
$ 3.18 = \dfrac{{4 \times 78}}{{6.023 \times {{10}^{23}} \times {a^3}}} $
$ {a^3} = \dfrac{{312}}{{6.023 \times {{10}^{23}} \times 3.18}} $
$ {a^3} = 16.28 \times {10^{ - 23}} $
$ a = 546{\text{pm}} $
Hence option c is correct.
Note:
Knowing the dimensions of the unit cell allows us to calculate the volume of the unit cell. For example, if an edge "a" has a unit cell, the volume of the unit cell may be written as " $ {a^3} $ " The density of a unit cell is defined as the ratio of its mass to its volume. The product of the number of atoms in a unit cell and the mass of each atom in a unit cell equals the mass of a unit cell.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




