
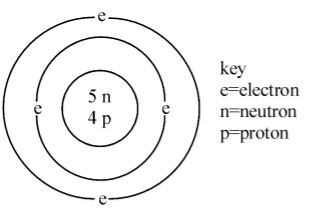
The diagram given below represents the atom of an element. Which symbol gives the above information?
A.${{_{4}}^{9}}Be$
B.${{_{4}}^{9}}F$
C.${{_{4}}^{9}}B$
D.${{_{4}}^{9}}O$

Answer
573.6k+ views
Hint: Atomic number is defined as the total no of protons or electrons present in the nucleus or in the orbits of the atoms.
Mass number is defined as the total no of neutrons and protons present in the nucleus of the atom.
Complete answer:
The term atomic number is conventionally denoted by the symbol Z it indicates the number of protons present in the nucleus of an atom, which is also equal to the number of electrons in an uncharged atom. The number of neutrons in the atom is represented by the neutron number (N). It is written below the element
Similarly the term mass no is conventionally denoted by the symbol M it indicates the no of protons and neutrons present in the nucleus of the atom. The number of neutrons is denoted by the mass no(M). It is written above the element.
In the above diagram there are 4 protons so the atomic no is 4 and written below.
And there are 5 neutrons and 4 protons ,there are a total 9 protons and neutrons so the mass no is 9 and written above.
As the element with atomic no 4 is beryllium.
Therefore the correct option is A.
Note:
Beryllium is a chemical element with the symbol Be and has an atomic number 4. It is an apparently rare element in the universe, usually occurring as a product of the disintegration of larger atomic nuclei that have collided with cosmic rays. Within the cores of stars, beryllium is depleted as it is fused into other heavier elements.
Mass number is defined as the total no of neutrons and protons present in the nucleus of the atom.
Complete answer:
The term atomic number is conventionally denoted by the symbol Z it indicates the number of protons present in the nucleus of an atom, which is also equal to the number of electrons in an uncharged atom. The number of neutrons in the atom is represented by the neutron number (N). It is written below the element
Similarly the term mass no is conventionally denoted by the symbol M it indicates the no of protons and neutrons present in the nucleus of the atom. The number of neutrons is denoted by the mass no(M). It is written above the element.
In the above diagram there are 4 protons so the atomic no is 4 and written below.
And there are 5 neutrons and 4 protons ,there are a total 9 protons and neutrons so the mass no is 9 and written above.
As the element with atomic no 4 is beryllium.
Therefore the correct option is A.
Note:
Beryllium is a chemical element with the symbol Be and has an atomic number 4. It is an apparently rare element in the universe, usually occurring as a product of the disintegration of larger atomic nuclei that have collided with cosmic rays. Within the cores of stars, beryllium is depleted as it is fused into other heavier elements.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




