
The diagram shows an atom of uranium-235 being split into several pieces. What is the name of the process shown in the diagram? What is the name of the particles labelled X?

Answer
531.3k+ views
Hint: We have given that the uranium-235 is being split into some dfractions i.e. this is the nuclear fission reaction forming some other radio-isotopic atoms such as Ba-144 and Kr-89.
We need to consider all the facts with respect to nuclear reactions while identifying the component ‘X’.
Complete answer:
Let us know all the facts related to nuclear reactions-
Mass is conserved i.e. overall mass number of reactants is equal to overall mass number of products.
Charge is conserved i.e. overall atomic number of reactants is equal to overall atomic number of reactants.
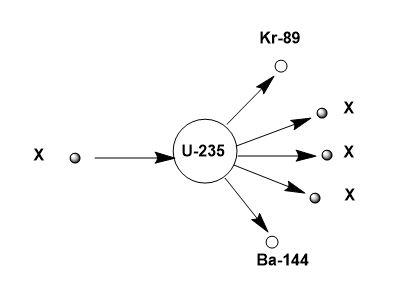
Now, looking at the diagram given;
The component labelled ‘X’ is present as; 1 quantity in the reactant side and 3 quantities in the product side.
The mass numbers of U = 235, Kr = 89 and Ba = 144.
Referring the modern periodic table, we know that,
Atomic numbers of U = 92, Kr = 36 and Ba = 56.
Now, according to the facts mentioned above;
Z + 92 = 36 + 56 + 3Z (conservation of charge); where, Z is the atomic number of ‘X’.
Thus, $Z=\dfrac{36+56-92}{-2}=0$
And,
A + 235 = 89 + 144 + 3A (conservation of mass); where A is the mass number of ‘X’.
Thus, $A=\dfrac{89+144-235}{-2}=\dfrac{-2}{-2}=1$
Therefore, you can say that the particle is a neutron as it no charge and has mass number 1 i.e.
\[{}_{Z}^{A}X={}_{0}^{1}n\]
The balanced nuclear reaction in accordance with the diagram will be;
${}_{0}^{1}n+{}_{92}^{235}U\to {}_{36}^{89}Kr+{}_{56}^{144}Ba+3{}_{0}^{1}n$
Note:
Do note the above reaction is nuclear fission reaction which follows two rules i.e. conservation of mass and charge. This reaction takes place with the release of immense energy along with other atoms.
Also, it’s a hint that every missing particle is labelled as ‘X’ i.e. every particle will be the same with respect to behaviour and properties.
We need to consider all the facts with respect to nuclear reactions while identifying the component ‘X’.
Complete answer:
Let us know all the facts related to nuclear reactions-
Mass is conserved i.e. overall mass number of reactants is equal to overall mass number of products.
Charge is conserved i.e. overall atomic number of reactants is equal to overall atomic number of reactants.
Now, looking at the diagram given;
The component labelled ‘X’ is present as; 1 quantity in the reactant side and 3 quantities in the product side.
The mass numbers of U = 235, Kr = 89 and Ba = 144.
Referring the modern periodic table, we know that,
Atomic numbers of U = 92, Kr = 36 and Ba = 56.
Now, according to the facts mentioned above;
Z + 92 = 36 + 56 + 3Z (conservation of charge); where, Z is the atomic number of ‘X’.
Thus, $Z=\dfrac{36+56-92}{-2}=0$
And,
A + 235 = 89 + 144 + 3A (conservation of mass); where A is the mass number of ‘X’.
Thus, $A=\dfrac{89+144-235}{-2}=\dfrac{-2}{-2}=1$
Therefore, you can say that the particle is a neutron as it no charge and has mass number 1 i.e.
\[{}_{Z}^{A}X={}_{0}^{1}n\]
The balanced nuclear reaction in accordance with the diagram will be;
${}_{0}^{1}n+{}_{92}^{235}U\to {}_{36}^{89}Kr+{}_{56}^{144}Ba+3{}_{0}^{1}n$
Note:
Do note the above reaction is nuclear fission reaction which follows two rules i.e. conservation of mass and charge. This reaction takes place with the release of immense energy along with other atoms.
Also, it’s a hint that every missing particle is labelled as ‘X’ i.e. every particle will be the same with respect to behaviour and properties.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




