
The dipole moments of $CC{l_4}$ , $CHC{l_3}$ and $C{H_4}$ are in the order :
A.$C{H_4} = CC{l_4} < CHC{l_3}$
B.$CC{l_4} < C{H_4} < CHC{l_3}$
C.$C{H_4} < CC{l_4} < CHC{l_3}$
D.$CHC{l_3} < C{H_4} = CC{l_4}$
Answer
582.6k+ views
Hint:The term dipole moment depends on various other concepts like polarity and pseudo charge. Apart from that the main factors affecting the dipole moment are difference in electrostatic charge and the structure of the given molecule. After making the structural diagrams for the given options we can observe that in among which of the diagrams there is least cancellation of the charges. And in among which we attain the most polarized ends. Then we can get the right answer.
Complete step by step answer:
Dipole moment is the phenomenon of the generation of the polarity in the structure of a specific structure of compound due to the generation of pseudo charge among the atoms of the molecule. This is mainly due to the difference in the electronegativity or electropositivity of various elements.
The generation of the dipole moment in a neutral environment is mainly due to $2$ reasons:
i.The first one is the difference in the electronegativity or electropositivity
ii.And the second one is the structure of the molecule.
For. Eg: the hydrogen would have no dipole moment due to the linear structure and no difference in electronegativity .
Now regarding the options :
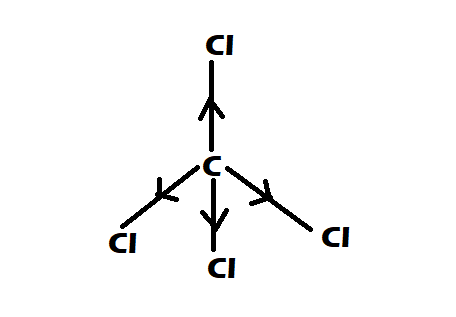
Option 1: $CC{l_4}$
-It is having perfect tetrahedral shape
-They have $0$ dipole moment
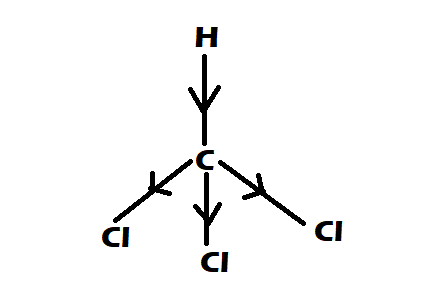
Option 2: $CHC{l_3}$
-It is having perfect tetrahedral shape
-They have $0$ dipole moment
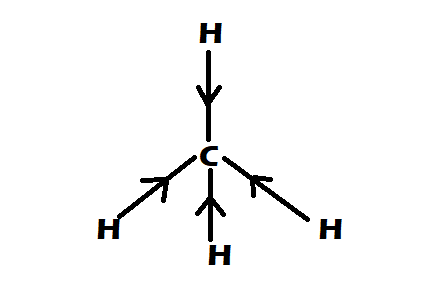
Option 3: $C{H_4}$
-It is having perfect tetrahedral shape
-They have $0$ dipole moment
Therefore the correct option would be option A, $C{H_4} = CC{l_4} < CHC{l_3}$ .
Note:Despite having the same atoms connected to the element many times the dipole moment don’t be null. This happens due to the change in the structure in which the charges don't cancel each other and this happens due to the presence of the lone pair.
Complete step by step answer:
Dipole moment is the phenomenon of the generation of the polarity in the structure of a specific structure of compound due to the generation of pseudo charge among the atoms of the molecule. This is mainly due to the difference in the electronegativity or electropositivity of various elements.
The generation of the dipole moment in a neutral environment is mainly due to $2$ reasons:
i.The first one is the difference in the electronegativity or electropositivity
ii.And the second one is the structure of the molecule.
For. Eg: the hydrogen would have no dipole moment due to the linear structure and no difference in electronegativity .
Now regarding the options :
Option 1: $CC{l_4}$
-It is having perfect tetrahedral shape
-They have $0$ dipole moment

Option 2: $CHC{l_3}$
-It is having perfect tetrahedral shape
-They have $0$ dipole moment

Option 3: $C{H_4}$
-It is having perfect tetrahedral shape
-They have $0$ dipole moment

Therefore the correct option would be option A, $C{H_4} = CC{l_4} < CHC{l_3}$ .
Note:Despite having the same atoms connected to the element many times the dipole moment don’t be null. This happens due to the change in the structure in which the charges don't cancel each other and this happens due to the presence of the lone pair.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




