
The distinction between conductors, insulators and semiconductors is largely concerned with
A. Their ability to conduct current
B. The type of crystal lattice
C. Binding energy of their electrons
D. Relative widths of their energy gaps
Answer
584.4k+ views
Hint: According to band theory of solids, the close interactions of the valence orbital of the crystal interactions occur. These interactions lead to the splitting in orbitals of same energy levels. They are namely: Conduction and Valence band. The difference in the energy level is called band or energy gap. The distinction ultimately depends on this factor.
Complete step by step answer:
According to electrical conductivity (σ) or resistivity(ρ) solids are classified into three kinds: Metals, Insulators and Semiconductors.
The distinction between these can be understood with the help of Band theory of Solids.
Normally, the electrons have discrete energies in different orbits when they exist separately. But in a crystal, these electrons are made to interact with each other.
This interaction is primarily felt by the valence electrons.
The orbitals of these valence electrons split into two bands, due to the interactions. They are the valence band and the conduction band. As shown in the graph.
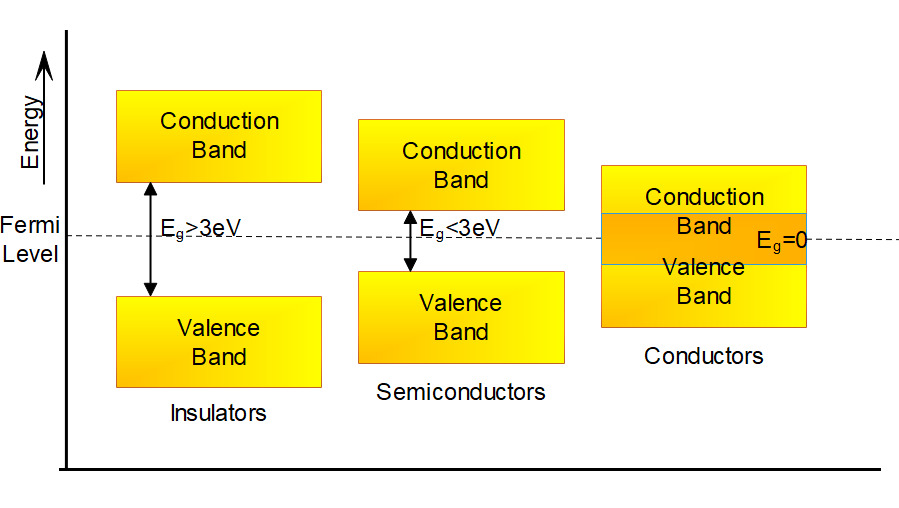
The band here denotes equal energy level. Thus, the valence band and conduction band are different levels of energy.
This difference in the energy levels is known as band gap or forbidden energy gap, ${E_g}$.
For conductors, the energy gap is absent i.e., ${E_g} = 0$. For insulators, ${E_g} > 3eV$.
While for Semiconductors, ${E_g} < 3eV$.
Thus, it all comes down to the energy gap.
Therefore, the correct option is D.
Note:
Conduction band contains free electrons which participate in conduction. Semiconductors have an empty conduction band while it is partially filled for conductors. It has higher energy compared to the valence band. Valence band contains valence electrons (i.e., outermost orbit electrons). For semiconductors this band may be completely filled. The energy at which the valence band and conduction band are closest to each other is called fermi level. The conduction band resides above the fermi level and the valence band; below.
Complete step by step answer:
According to electrical conductivity (σ) or resistivity(ρ) solids are classified into three kinds: Metals, Insulators and Semiconductors.
The distinction between these can be understood with the help of Band theory of Solids.
Normally, the electrons have discrete energies in different orbits when they exist separately. But in a crystal, these electrons are made to interact with each other.
This interaction is primarily felt by the valence electrons.
The orbitals of these valence electrons split into two bands, due to the interactions. They are the valence band and the conduction band. As shown in the graph.

The band here denotes equal energy level. Thus, the valence band and conduction band are different levels of energy.
This difference in the energy levels is known as band gap or forbidden energy gap, ${E_g}$.
For conductors, the energy gap is absent i.e., ${E_g} = 0$. For insulators, ${E_g} > 3eV$.
While for Semiconductors, ${E_g} < 3eV$.
Thus, it all comes down to the energy gap.
Therefore, the correct option is D.
Note:
Conduction band contains free electrons which participate in conduction. Semiconductors have an empty conduction band while it is partially filled for conductors. It has higher energy compared to the valence band. Valence band contains valence electrons (i.e., outermost orbit electrons). For semiconductors this band may be completely filled. The energy at which the valence band and conduction band are closest to each other is called fermi level. The conduction band resides above the fermi level and the valence band; below.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




