
The electrolytic reduction of nitrobenzene in strongly acidic medium produces:
A. Azobenzene
B. Aniline
C. p-Aminophenol
D. Azoxy benzene
Answer
520.8k+ views
Hint: Electrolytic reduction is an electrolysis in which current is passed through an ionic substance producing chemical reactions at the electrodes (positive and negative) and decomposing the original substance. Acids like Hydrochloric acid ($HCl$), Sulphuric acid (${H_2}S{O_4}$) are used for strongly acidic mediums.
Step by step answer:
We are given to find the product of electrolytic reduction of nitrobenzene in a strongly acidic medium.
Electrolytic reduction is a type of electrolysis of a substance in presence of other ionic substances in which electric current is passed through it to decompose the substance into new compounds.
Nitrobenzene is an oily, aromatic nitro-compound that emits toxic fumes of nitrogen oxides upon combustion with a chemical formula ${C_6}{H_5}N{O_2}$.
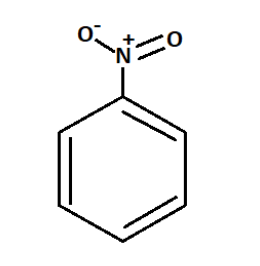
We know that in strongly acidic medium, Sulphuric acid is used as the strong acid.
So when nitrobenzene reacts with sulphuric acid it produces Phenyl hydroxylamine.
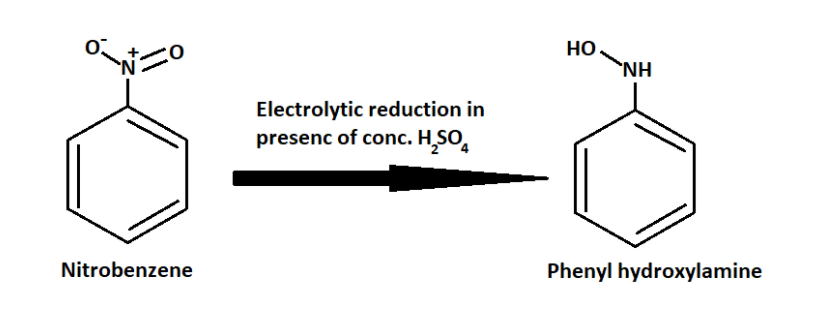
And then the above Phenyl hydroxylamine rearranges to form p- Aminophenol.
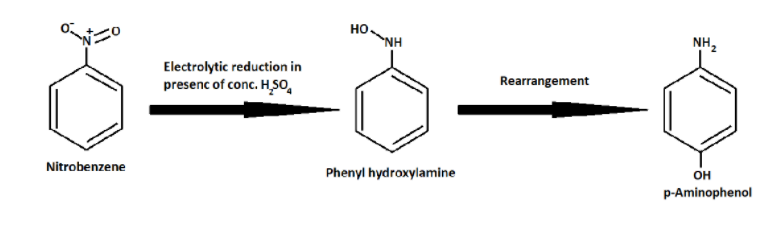
In a weakly acidic medium, nitrobenzene on electrolytic reduction gives aniline but under strongly acidic medium it gives p-aminophenol.
In the alkaline medium, various mono and di-nuclear reduction products such as Azoxybenzene, Azobenzene are obtained.
So when nitrobenzene in the presence of strong acidic medium on electrolysis, gives p-Aminophenol
Therefore, the correct option is Option C, p-Aminophenol.
Additional Information:
Nitrobenzene is used to produce a chemical called aniline; to produce lubricating oils used in motors and machines; to manufacture drugs, pesticides etc.
Note: Benzene is an organic compound (hydro-carbon) with six carbons and six hydrogens. Six carbon atoms are joined in a planar ring with one hydrogen atom attached to each carbon atom. Nitrobenzene is obtained by replacing one of the hydrogens with Nitrogen dioxide. Be careful with the oxidation numbers of nitrogen and oxygen in nitrogen dioxide
Step by step answer:
We are given to find the product of electrolytic reduction of nitrobenzene in a strongly acidic medium.
Electrolytic reduction is a type of electrolysis of a substance in presence of other ionic substances in which electric current is passed through it to decompose the substance into new compounds.
Nitrobenzene is an oily, aromatic nitro-compound that emits toxic fumes of nitrogen oxides upon combustion with a chemical formula ${C_6}{H_5}N{O_2}$.

We know that in strongly acidic medium, Sulphuric acid is used as the strong acid.
So when nitrobenzene reacts with sulphuric acid it produces Phenyl hydroxylamine.

And then the above Phenyl hydroxylamine rearranges to form p- Aminophenol.

In a weakly acidic medium, nitrobenzene on electrolytic reduction gives aniline but under strongly acidic medium it gives p-aminophenol.
In the alkaline medium, various mono and di-nuclear reduction products such as Azoxybenzene, Azobenzene are obtained.
So when nitrobenzene in the presence of strong acidic medium on electrolysis, gives p-Aminophenol
Therefore, the correct option is Option C, p-Aminophenol.
Additional Information:
Nitrobenzene is used to produce a chemical called aniline; to produce lubricating oils used in motors and machines; to manufacture drugs, pesticides etc.
Note: Benzene is an organic compound (hydro-carbon) with six carbons and six hydrogens. Six carbon atoms are joined in a planar ring with one hydrogen atom attached to each carbon atom. Nitrobenzene is obtained by replacing one of the hydrogens with Nitrogen dioxide. Be careful with the oxidation numbers of nitrogen and oxygen in nitrogen dioxide
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




