
The electronic structure of the $S{{O}_{2}}$ molecule is best represented as a resonance hybrid of
Equivalent structures.
A) 4
B) 2
C) This molecule does not exhibit resonance
D) 3
Answer
566.7k+ views
Hint: In $S{{O}_{2}}$ molecule there are two types of pi-bonds.
Total number of valence electrons we have are 18 electrons.6 electrons from S atom and 6 electrons from each O atom.
- S atoms possess a positive charge in their equivalent structure.
Complete Solution :
So the question is about that number of resonance structure that supports the actual structure of $S{{O}_{2}}$ molecule and helps to explain every property of the molecule.
- To draw resonance structure of $S{{O}_{2}}$, we have 18 ${{e}^{-}}$ and there is positive charge in S atom and negative charge in one of the O atom in the resonance structure.
- Actually there are three Lewis structures for $S{{O}_{2}}$ molecules, but only two of them satisfy the experimental data and the third one only supports the theoretical part.
Now let’s draw the resonance structure of $S{{O}_{2}}$
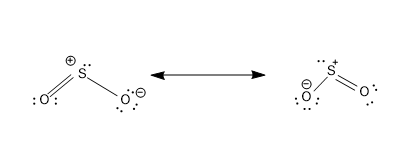
- These structures have a count of 18${{e}^{-}}$, i.e 6 ${{e}^{-}}$ from three bonds and 12${{e}^{-}}$ are distributed as lone pairs in three atoms.
- The structures consist of proper formal charges –Negative formal charge on the most electronegative O atom and positive formal charge on the comparatively less electronegative S atom.
- These two structures are equivalent and will equally contribute to the hybrid structure of $S{{O}_{2}}$
In that case the hybrid structure must be
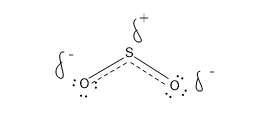
Here there is a partial negative charge on the O and a partial positive charge on the S.
The negative charge is split between two O.
So, the correct answer is “Option B”.
Note: The third Lewis structure of $S{{O}_{2}}$ is,

This structure is stable for the theory aspect, but does not produce evidence for experimental data.
Total number of valence electrons we have are 18 electrons.6 electrons from S atom and 6 electrons from each O atom.
- S atoms possess a positive charge in their equivalent structure.
Complete Solution :
So the question is about that number of resonance structure that supports the actual structure of $S{{O}_{2}}$ molecule and helps to explain every property of the molecule.
- To draw resonance structure of $S{{O}_{2}}$, we have 18 ${{e}^{-}}$ and there is positive charge in S atom and negative charge in one of the O atom in the resonance structure.
- Actually there are three Lewis structures for $S{{O}_{2}}$ molecules, but only two of them satisfy the experimental data and the third one only supports the theoretical part.
Now let’s draw the resonance structure of $S{{O}_{2}}$

- These structures have a count of 18${{e}^{-}}$, i.e 6 ${{e}^{-}}$ from three bonds and 12${{e}^{-}}$ are distributed as lone pairs in three atoms.
- The structures consist of proper formal charges –Negative formal charge on the most electronegative O atom and positive formal charge on the comparatively less electronegative S atom.
- These two structures are equivalent and will equally contribute to the hybrid structure of $S{{O}_{2}}$
In that case the hybrid structure must be

Here there is a partial negative charge on the O and a partial positive charge on the S.
The negative charge is split between two O.
So, the correct answer is “Option B”.
Note: The third Lewis structure of $S{{O}_{2}}$ is,

This structure is stable for the theory aspect, but does not produce evidence for experimental data.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




