
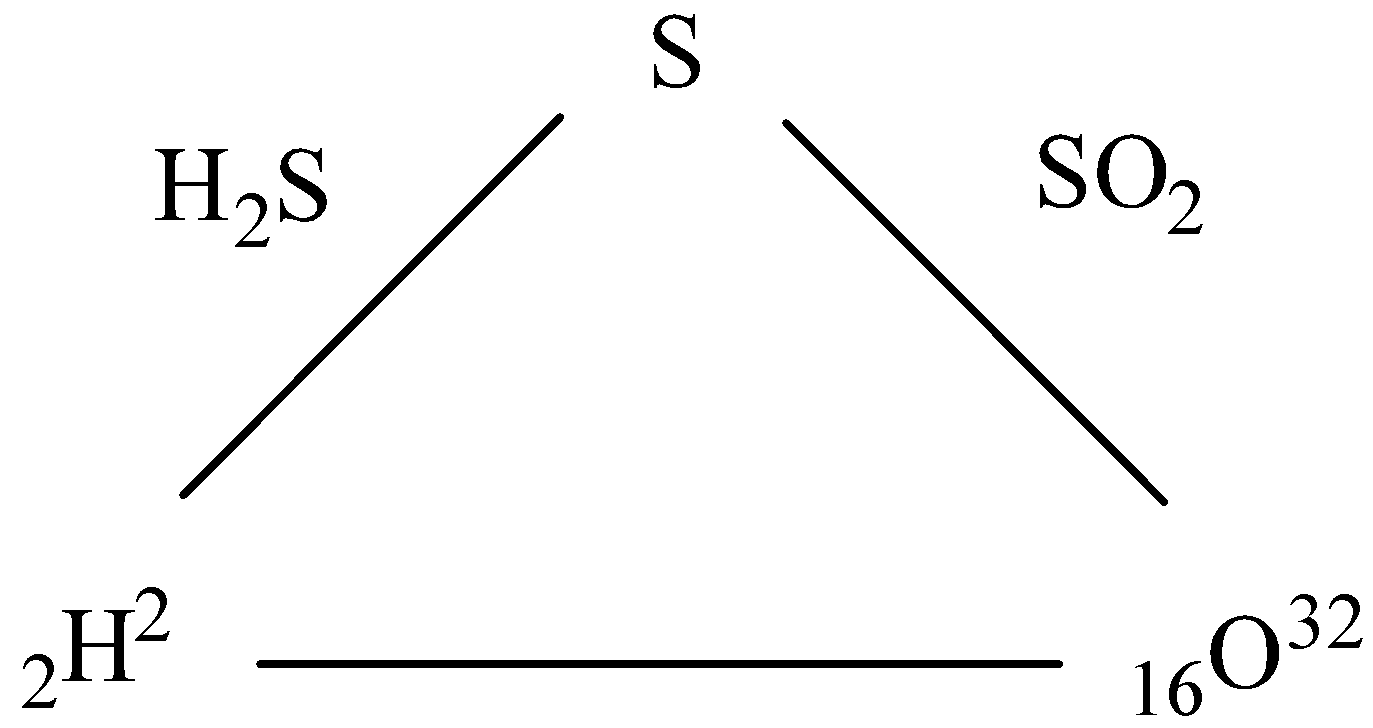
The element H and O combine separately with the third element S to form ${H_2}S$ and $S{O_2}$ respectively, then show that they combine directly with each other to form ${H_2}O$.

Answer
573.6k+ views
Hint: Law of reciprocal proportion is also called the law of equivalent proportions. This law states that if two different atoms combine with a fixed mass of a third element separately, then the ratio of masses in which they combine is also the same or multiple of the ratio of masses obtained.
Complete step by step solution:
To predict how O-atom and H-atom will combine to form ${H_2}O$ from the given data can be predicted by the rule of reciprocal proportion.
- Law of reciprocal proportion is also called law of equivalent proportions.
- This law states that if two different atoms combine with a fixed mass of a third element separately, then the ratio of masses in which they combine is also the same or multiple of the ratio of masses obtained.
- It is given that H atom and S atom combine to give ${H_2}S$ . So, as the atomic weight of H is 2 $gmo{l^{ - 1}}$ and that of S is 32 $gmo{l^{ - 1}}$. So, we can say that 2 g of H atoms can combine with 32 g of S atoms.
So, the ratio of weight of H atoms to S atoms is 1:16.
Now, We know that the atomic weight of an O atom is 16$gmo{l^{ - 1}}$.
In $S{O_2}$, we can see that 32 g of S atoms combine with 16$ \times $ 2 = 32g of O atoms. So, the ratio of weight of S atoms to O atoms in the given compound is 1:1.
So, we can say from the above ratio obtained that H atom and O atom should combine in a way that the ratio of H atoms to O atoms will be multiple of $\dfrac{{16}}{2} = 8$ g.
Thus, we can say that 1 g of H atoms can combine with 8 g of o atoms.
- In ${H_2}O$, we can see that the mass of H atoms is 2 g and that of O atom is 16 gm. So, the ratio of masses of atoms can be given by 1:8.
Note: The Law of proportions was proposed first by Jeremias Ritcher. With the acceptance of this law, the equivalent weights of elements were drawn up. The law of multiple proportions is different from this law as it describes the stoichiometric relation between two atoms in different compounds.
Complete step by step solution:
To predict how O-atom and H-atom will combine to form ${H_2}O$ from the given data can be predicted by the rule of reciprocal proportion.
- Law of reciprocal proportion is also called law of equivalent proportions.
- This law states that if two different atoms combine with a fixed mass of a third element separately, then the ratio of masses in which they combine is also the same or multiple of the ratio of masses obtained.
- It is given that H atom and S atom combine to give ${H_2}S$ . So, as the atomic weight of H is 2 $gmo{l^{ - 1}}$ and that of S is 32 $gmo{l^{ - 1}}$. So, we can say that 2 g of H atoms can combine with 32 g of S atoms.
So, the ratio of weight of H atoms to S atoms is 1:16.
Now, We know that the atomic weight of an O atom is 16$gmo{l^{ - 1}}$.
In $S{O_2}$, we can see that 32 g of S atoms combine with 16$ \times $ 2 = 32g of O atoms. So, the ratio of weight of S atoms to O atoms in the given compound is 1:1.
So, we can say from the above ratio obtained that H atom and O atom should combine in a way that the ratio of H atoms to O atoms will be multiple of $\dfrac{{16}}{2} = 8$ g.
Thus, we can say that 1 g of H atoms can combine with 8 g of o atoms.
- In ${H_2}O$, we can see that the mass of H atoms is 2 g and that of O atom is 16 gm. So, the ratio of masses of atoms can be given by 1:8.
Note: The Law of proportions was proposed first by Jeremias Ritcher. With the acceptance of this law, the equivalent weights of elements were drawn up. The law of multiple proportions is different from this law as it describes the stoichiometric relation between two atoms in different compounds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




