
The empirical formula of octane \[{{C}_{8}}{{H}_{18}}\] is:
(a) \[{{C}_{4}}{{H}_{9}}\]
(b) \[{{C}_{2}}{{H}_{9}}\]
(c) \[{{C}_{8}}{{H}_{18}}\]
(d) \[{{C}_{3}}{{H}_{13}}\]
Answer
602.1k+ views
Hint: Empirical formula is the smallest ratio of elements present in the compound. So, try to solve this by finding out the simplest ratio of carbon and hydrogen in octane. Do not confuse empirical formula with molecular formula.
Complete step by step answer:
We can break the term ‘Octane’ into two parts, i.e. ‘oct-’ + ‘-ane’.
‘oct-’ symbolizes that it is an eight-carbon compound.
‘-ane’ symbolizes that it is an alkane.
Its molecular formula is \[{{C}_{8}}{{H}_{18}}\]
The structure of octane can be drawn as –
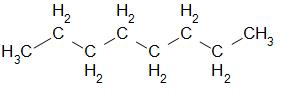
Now let us understand the meaning of empirical formula.
The empirical formula of any chemical compound is defined as the simplest positive integer ratio (or whole number ratio) of atoms present in a compound.
As we can see, the ratio of carbon: hydrogen = 8: 18
If we divide this by 2, we will obtain the simplest ratio of the compound, i.e. empirical formula.
Simple ratio = 4: 9
Empirical formula = \[{{C}_{4}}{{H}_{9}}\]
Therefore, the answer is – option (a) – The empirical formula of octane \[{{C}_{8}}{{H}_{18}}\] is \[{{C}_{4}}{{H}_{9}}\].
Additional Information:
Octane is one of the main components of gasoline (petrol), it is because octane is volatile and very flammable in nature.
Note: The molecular formula of a compound can be calculated using the empirical formula if the formula weight, or molecular weight, is known. It can also be calculated by percentage weight of the elements in a compound.
Complete step by step answer:
We can break the term ‘Octane’ into two parts, i.e. ‘oct-’ + ‘-ane’.
‘oct-’ symbolizes that it is an eight-carbon compound.
‘-ane’ symbolizes that it is an alkane.
Its molecular formula is \[{{C}_{8}}{{H}_{18}}\]
The structure of octane can be drawn as –

Now let us understand the meaning of empirical formula.
The empirical formula of any chemical compound is defined as the simplest positive integer ratio (or whole number ratio) of atoms present in a compound.
As we can see, the ratio of carbon: hydrogen = 8: 18
If we divide this by 2, we will obtain the simplest ratio of the compound, i.e. empirical formula.
Simple ratio = 4: 9
Empirical formula = \[{{C}_{4}}{{H}_{9}}\]
Therefore, the answer is – option (a) – The empirical formula of octane \[{{C}_{8}}{{H}_{18}}\] is \[{{C}_{4}}{{H}_{9}}\].
Additional Information:
Octane is one of the main components of gasoline (petrol), it is because octane is volatile and very flammable in nature.
Note: The molecular formula of a compound can be calculated using the empirical formula if the formula weight, or molecular weight, is known. It can also be calculated by percentage weight of the elements in a compound.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




