
The ester \[RCOOR'\] can be prepared by:
(this question has multiple correct options)
A.Esterification of RCOOH
B.Esterification of \[{(RCO)_2}O\]
C.Baeyer Villager oxidation of \[RCOR'\] with a peroxy acid
D.Reaction of \[R'COCl\] with ROH
Answer
583.5k+ views
Hint: Esters are basically substituted products of carboxylic acids. Esters are formed when the hydrogen from the \[ - OH\] group in the carboxylic acid functional group is replaced by an aryl or alkyl functional group.
Complete step by step answer:
To understand the adequate processes that can produce esters from the options mentioned above, we must first understand each process and then derive conclusions.
Esterification of RCOOH or carboxylic acids is a process which involves a reaction between carboxylic acid and alcohol. This reaction results in the formation of an ester and water.
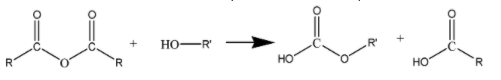
\[{(RCO)_2}O\] is the simplified representation of acid anhydrides. The process of esterification of \[{(RCO)_2}O\] involves the reaction of an acid anhydride with an alcohol. This reaction results in
the formation of an ester and a carboxylic acid. . It can be represented as follows:
\[RCOR'\] is the simplified representation of ketones. In Baeyer - Villager oxidation, a ketone uses peroxides as catalyst and forms an ester.
\[RCOR'\xrightarrow{{peroxide}}RCOOR'\]
is the simplified name for acyl chloride. When acyl chlorides are reacted with alcohols, they result in the formation of hydrochloric acid and a corresponding ester.
\[R'COCl{\text{ }} + {\text{ }}ROH\;RCOOR'{\text{ }} + {\text{ }}HCl\]
Hence, options A, B, C and D are the correct options.
Additional information:
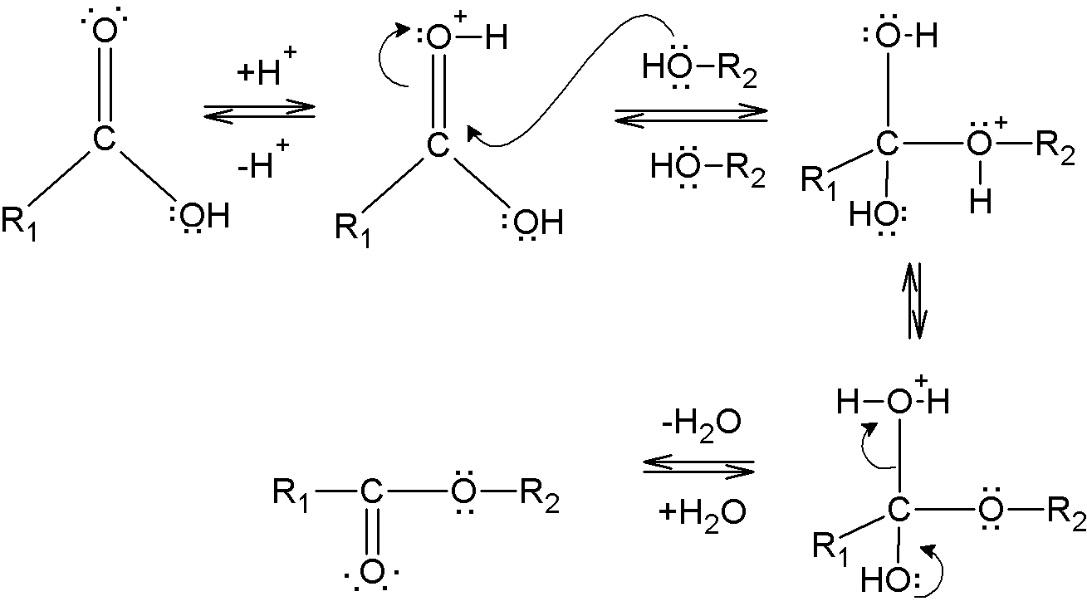
The basis for an esterification reaction can be represented as follows –
Note:
All the processes above are all basic forms of substitution reactions. Esterification or formation esters usually takes place because of these types of substitution reactions. Also, the formation of simple esters is done through a process known as Fisher esterification. This reaction involves the conversion of a carboxylic acid and an alcohol into an ester while forming water as a by-product. The method of Fisher esterification is a reversible reaction, hence this reaction proceeds very slowly.
Complete step by step answer:
To understand the adequate processes that can produce esters from the options mentioned above, we must first understand each process and then derive conclusions.
Esterification of RCOOH or carboxylic acids is a process which involves a reaction between carboxylic acid and alcohol. This reaction results in the formation of an ester and water.
\[{(RCO)_2}O\] is the simplified representation of acid anhydrides. The process of esterification of \[{(RCO)_2}O\] involves the reaction of an acid anhydride with an alcohol. This reaction results in
the formation of an ester and a carboxylic acid. . It can be represented as follows:

\[RCOR'\] is the simplified representation of ketones. In Baeyer - Villager oxidation, a ketone uses peroxides as catalyst and forms an ester.
\[RCOR'\xrightarrow{{peroxide}}RCOOR'\]
is the simplified name for acyl chloride. When acyl chlorides are reacted with alcohols, they result in the formation of hydrochloric acid and a corresponding ester.
\[R'COCl{\text{ }} + {\text{ }}ROH\;RCOOR'{\text{ }} + {\text{ }}HCl\]
Hence, options A, B, C and D are the correct options.
Additional information:
The basis for an esterification reaction can be represented as follows –

Note:
All the processes above are all basic forms of substitution reactions. Esterification or formation esters usually takes place because of these types of substitution reactions. Also, the formation of simple esters is done through a process known as Fisher esterification. This reaction involves the conversion of a carboxylic acid and an alcohol into an ester while forming water as a by-product. The method of Fisher esterification is a reversible reaction, hence this reaction proceeds very slowly.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




