
The fibre obtained by the condensation of hexamethylenediamine and adipic acid is:
1.Dacron
2.Nylon-6,6
3.Rayon
4.Teflon
Answer
568.2k+ views
Hint:
In the condensation polymerization reaction, two molecules are combining each other to form one single molecule by the elimination of simple molecules like water. A functional group interacts with the group on another monomer at one end of a monomer, resulting in long chain condensation polymers.
Complete step by step answer:
Hexamethylenediamine and adipic acid are copolymers that undergo condensation polymerization reactions to form a nylon salt by removing water molecules.
The following chemical reaction is shown in the formation of Nylon -6,6.
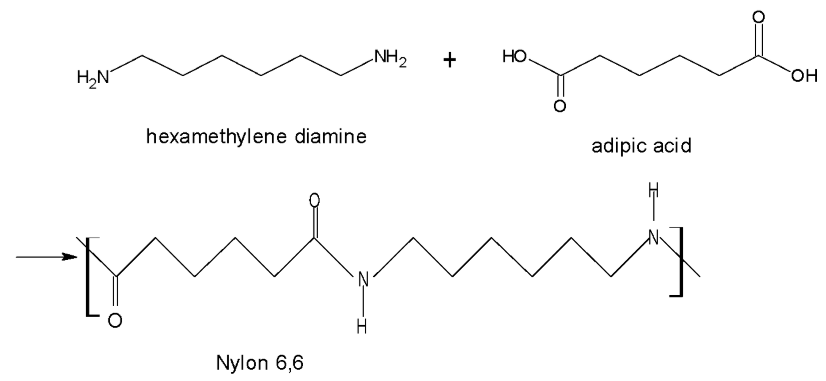
Hence, the correct option is (2).
Additional information:
Adipic Acid is a crystalline solid that is colourless and used in nylon preparation. It is insoluble in water but slightly soluble in acetone.
Hexamethylene diamine is present in the saturated form and it is a main compound to use in the production of polymers like nylon and other plastics. And it is a colourless crystalline solid.
DACRON – It is a thermoplastic and the chemical name of the compound is polyethylene terephthalate.
RAYON – The main ingredient to formation of this polymer is cellulose; it is a naturally occurring polymer. And it is a semi-synthetic polymer.
TEFLON - It is formed by addition of n number of tetrafluoro ethene. High mechanical strength, stiffness, hardness and toughness. Excellent wear resistance. Good electrical insulating properties.
Note:Nylon 6,6 is a polyamide and synthetic fibre and it is most resistant to heat and friction, commonly used in textiles and plastic industries. It has a dense structure with pores that are small and uniformly distributed. This implies that it is difficult to dye nylon 6,6, but it has superior colour fastness once dyed and is less susceptible to sunlight and ozone fading.
In the condensation polymerization reaction, two molecules are combining each other to form one single molecule by the elimination of simple molecules like water. A functional group interacts with the group on another monomer at one end of a monomer, resulting in long chain condensation polymers.
Complete step by step answer:
Hexamethylenediamine and adipic acid are copolymers that undergo condensation polymerization reactions to form a nylon salt by removing water molecules.
The following chemical reaction is shown in the formation of Nylon -6,6.

Hence, the correct option is (2).
Additional information:
Adipic Acid is a crystalline solid that is colourless and used in nylon preparation. It is insoluble in water but slightly soluble in acetone.
Hexamethylene diamine is present in the saturated form and it is a main compound to use in the production of polymers like nylon and other plastics. And it is a colourless crystalline solid.
DACRON – It is a thermoplastic and the chemical name of the compound is polyethylene terephthalate.
RAYON – The main ingredient to formation of this polymer is cellulose; it is a naturally occurring polymer. And it is a semi-synthetic polymer.
TEFLON - It is formed by addition of n number of tetrafluoro ethene. High mechanical strength, stiffness, hardness and toughness. Excellent wear resistance. Good electrical insulating properties.
Note:Nylon 6,6 is a polyamide and synthetic fibre and it is most resistant to heat and friction, commonly used in textiles and plastic industries. It has a dense structure with pores that are small and uniformly distributed. This implies that it is difficult to dye nylon 6,6, but it has superior colour fastness once dyed and is less susceptible to sunlight and ozone fading.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




