
The first member of alkane homologous series is:
A)Ethyne
B)Ethene
C)Propyne
D)Methane
Answer
585.3k+ views
Hint: Homologous series of compounds are the series of compounds that have the same general formula but they differ from adjacent members by \[ - C{H_2}\] group and we can determine them by the hydrocarbon chain. Hydrocarbons include alkanes, alkenes, alkynes, etc. Alkanes have carbon atoms attached to other carbon atoms via single bonds.
Complete step-by-step answer:
We know that hydrocarbons such as alkanes, alkenes, and alkynes are a group of compounds which have only two constituents, carbon and hydrogen. In these, the carbon atoms are bonded through single bonds in alkanes, double bonds in alkenes and triple bonds in alkynes.
The homologous group of compounds have the same general formula, such as the general formula of homologous series alkanes is \[{C_n}{H_{2n + 2}}\] where n is the number of carbon atoms in the series. On putting the value of \[n = 1\] , we get its fist member i.e. \[C{H_4}\] (methane). We call them saturated homologous series as they have only single bonds. The structure of methane is
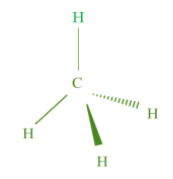
The homologous series of the same functional group and its substituents for hydrogen in a carbon chain and the common difference between a successive member of the family is \[ - C{H_2}\] . So, putting the values of n in the general formula, we can find out any member of that series.
Similarly, we can determine the first member of alkenes from the general formula \[{C_n}{H_{2n}}\] and alkynes from \[{C_n}{H_{2n - 2}}\] .
Hence, the correct option is (D).
Note: In alkanes, the first member has only one carbon atom, but in case of alkene and alkynes, the first member of both have two carbon atoms because they involve double and triple bond respectively which is not possible foe a single carbon. As the molecular mass increases in homologous series, change in physical properties observed as boiling and melting point also increases.
Complete step-by-step answer:
We know that hydrocarbons such as alkanes, alkenes, and alkynes are a group of compounds which have only two constituents, carbon and hydrogen. In these, the carbon atoms are bonded through single bonds in alkanes, double bonds in alkenes and triple bonds in alkynes.
The homologous group of compounds have the same general formula, such as the general formula of homologous series alkanes is \[{C_n}{H_{2n + 2}}\] where n is the number of carbon atoms in the series. On putting the value of \[n = 1\] , we get its fist member i.e. \[C{H_4}\] (methane). We call them saturated homologous series as they have only single bonds. The structure of methane is

The homologous series of the same functional group and its substituents for hydrogen in a carbon chain and the common difference between a successive member of the family is \[ - C{H_2}\] . So, putting the values of n in the general formula, we can find out any member of that series.
Similarly, we can determine the first member of alkenes from the general formula \[{C_n}{H_{2n}}\] and alkynes from \[{C_n}{H_{2n - 2}}\] .
Hence, the correct option is (D).
Note: In alkanes, the first member has only one carbon atom, but in case of alkene and alkynes, the first member of both have two carbon atoms because they involve double and triple bond respectively which is not possible foe a single carbon. As the molecular mass increases in homologous series, change in physical properties observed as boiling and melting point also increases.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




