
Write a brief note on hyperconjugation and give all hyper conjugated structures of propene.
Answer
599.7k+ views
Hint- Proceed the solution of this question using the concept of hyper-conjugation, which is a special case of resonance that involves delocalisation of σ electrons of C−H bond of any alkyl group. Hence, in the same way show the delocalisation of σ electrons of C−H bond in propene structure.
Complete answer:
Hyper-conjugation is the interaction of the electrons in a σ bond with an adjacent empty or partially filled non-bonding p-orbital, antibonding σ or π orbital or filled π orbital to give an extended molecular orbital that increases the stability of the system.
In case of propene, hyper conjugation arise due to partial overlap of ${\text{s}}{{\text{p}}^3}$ sigma bond orbital and the empty p-orbital of an adjacent c−atom.
Here one of the C-atom C−H bonds of $ - {\text{C}}{{\text{H}}_3}$ group can lie in the plane of pi-bond orbital, hence partial overlapping.
Hyper conjugation structures of propene are given below-
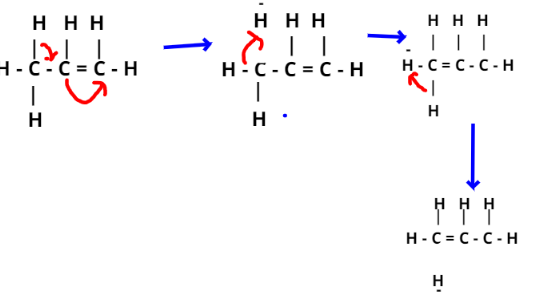
Note- In this particular question, we should know that Hyperconjugation is also known as no bond resonance. You may note that resonance involves delocalisation of π electrons but it’s the σ electrons that are delocalised in hyper conjugation. The normal carbon-carbon single bond length is \[1.54{\text{ }}{{\text{A}}^0}\]. However, in some compounds such as propene, it is a little shorter because of presence of some double bond character in C−C single bond due to hyper conjugation
Complete answer:
Hyper-conjugation is the interaction of the electrons in a σ bond with an adjacent empty or partially filled non-bonding p-orbital, antibonding σ or π orbital or filled π orbital to give an extended molecular orbital that increases the stability of the system.
In case of propene, hyper conjugation arise due to partial overlap of ${\text{s}}{{\text{p}}^3}$ sigma bond orbital and the empty p-orbital of an adjacent c−atom.
Here one of the C-atom C−H bonds of $ - {\text{C}}{{\text{H}}_3}$ group can lie in the plane of pi-bond orbital, hence partial overlapping.
Hyper conjugation structures of propene are given below-

Note- In this particular question, we should know that Hyperconjugation is also known as no bond resonance. You may note that resonance involves delocalisation of π electrons but it’s the σ electrons that are delocalised in hyper conjugation. The normal carbon-carbon single bond length is \[1.54{\text{ }}{{\text{A}}^0}\]. However, in some compounds such as propene, it is a little shorter because of presence of some double bond character in C−C single bond due to hyper conjugation
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




