
The following reaction is known as:
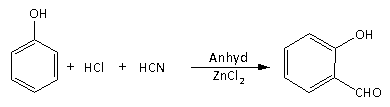
A) Gattermann reaction
B) Kolbe’s reaction
C) Perkin reaction
D) Gattermann-Koch Formylation

Answer
559.2k+ views
Hint:The formylation reactions are the reaction in which the formyl group \[{\text{ - CHO}}\]is introduced in the compound.Here, in the reaction, anhydrous zinc chloride is given which is Lewis acid, and acts as a catalyst for the reaction.Catalyst is the substance that increases the rate of the reaction without consuming the reaction.
Complete answer:
Here, option(A) Gattermann reaction.
It is the reaction in which formation of the aromatic compound is carried out using the mixture of HCl and HCN in presence of the Lewis base.
Here, in the given reaction phenol is converted into salicylic acid using the mixture of HCl and HCN in presence of the anhydrous zinc chloride.
Here, in option (B) Kolbe’s reaction is given. In this reaction sodium phenoxide reacts with carbon dioxide first, forming sodium salicylate which on further reaction gives the salicylic acid as a product.
Therefore, option(B) is an incorrect answer.
Option(C) Perkin reaction.
The reaction in which alpha, beta-unsaturated acid is prepared using the aldol condensation of the aromatic aldehyde and acid anhydride.
Therefore, option(C) is incorrect.
Option(D) Gattermann Koch Formylation.
It is the reaction in which formation of the aromatic compound is carried out using the mixture of CO and HCN in presence of the Lewis base like aluminum chloride.
Therefore, option(D) is incorrect.
Therefore, option(A) is the correct answer.
Note:Here, in the options, all given are the name reactions. Each name reaction is a specific reagent and is used.In the given reaction phenol is converted into salicylic acid in presence of the mixture of hydrochloric acid and hydrogen cyanide using zinc chloride which acts as a catalyst.
Thus, in this reaction reagent is used as a mixture of the HCl and HCN.
Complete answer:
Here, option(A) Gattermann reaction.
It is the reaction in which formation of the aromatic compound is carried out using the mixture of HCl and HCN in presence of the Lewis base.
Here, in the given reaction phenol is converted into salicylic acid using the mixture of HCl and HCN in presence of the anhydrous zinc chloride.
Here, in option (B) Kolbe’s reaction is given. In this reaction sodium phenoxide reacts with carbon dioxide first, forming sodium salicylate which on further reaction gives the salicylic acid as a product.
Therefore, option(B) is an incorrect answer.
Option(C) Perkin reaction.
The reaction in which alpha, beta-unsaturated acid is prepared using the aldol condensation of the aromatic aldehyde and acid anhydride.
Therefore, option(C) is incorrect.
Option(D) Gattermann Koch Formylation.
It is the reaction in which formation of the aromatic compound is carried out using the mixture of CO and HCN in presence of the Lewis base like aluminum chloride.
Therefore, option(D) is incorrect.
Therefore, option(A) is the correct answer.
Note:Here, in the options, all given are the name reactions. Each name reaction is a specific reagent and is used.In the given reaction phenol is converted into salicylic acid in presence of the mixture of hydrochloric acid and hydrogen cyanide using zinc chloride which acts as a catalyst.
Thus, in this reaction reagent is used as a mixture of the HCl and HCN.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




