
The formation of cyanohydrin from ketone is an example of:
[A] Electrophilic addition
[B] Nucleophilic addition
[C] Nucleophilic substitution
[D] Electrophilic addition
Answer
593.7k+ views
Hint:
To form cyanohydrin from ketone, as we can assume from the name itself, we will need a cyanide group. Cyanide can act as a nucleophile. As –OH is a bad leaving group, there will be no removal of a leaving group.
Complete step by step answer:
Before going into the formation of cyanohydrin from ketone, we will discuss what electrophilic and nucleophilic addition and substitution reactions are.
We know that electrophiles are electron deficient species i.e. they can accept a pair of electrons readily and nucleophiles are electron rich species i.e. they can donate a pair of electrons.
Electrophilic addition is the addition reaction where the group which is being added is an electrophile i.e. accepts a pair of electrons and nucleophilic addition is the addition reaction where the attacking group is a nucleophile i.e. it will donate a pair of electrons.
Similarly, electrophilic substitution is a reaction where the leaving group is substituted by an electrophile and nucleophilic substitution is a substitution reaction where the leaving group is replaced by the nucleophile by attacking on the positively charged atom to which the leaving group is attached.
To form cyanohydrin from ketone, we need a ketone and hydrogen cyanide.
The cyanide ion acts as a nucleophile which forms a bond with the electrophilic carbon centre of the ketone forming hydroxyacetonitrile which is commonly known as cyanohydrin. We can draw the mechanism of the reaction as-
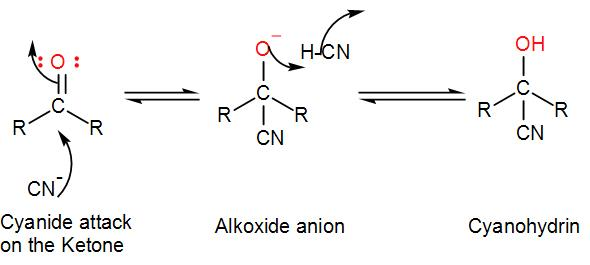
The alkoxide anion is protonated which regenerates the cyanide ion to continue the reaction.
Here, as we can see a nucleophile attacks the electrophilic centre and as there is no substitution, it is just addition therefore, this reaction is a nucleophilic addition.
Therefore, the correct answer is option [B] Nucleophilic addition.
Note:
As hydrogen cyanide is an acid itself, it will need a base to take its proton which will leave behind a cyanide anion. The carbon centre of the cyanide anion will act as a nucleophile. If base is absent, the reaction proceeds at a very slow rate.
To form cyanohydrin from ketone, as we can assume from the name itself, we will need a cyanide group. Cyanide can act as a nucleophile. As –OH is a bad leaving group, there will be no removal of a leaving group.
Complete step by step answer:
Before going into the formation of cyanohydrin from ketone, we will discuss what electrophilic and nucleophilic addition and substitution reactions are.
We know that electrophiles are electron deficient species i.e. they can accept a pair of electrons readily and nucleophiles are electron rich species i.e. they can donate a pair of electrons.
Electrophilic addition is the addition reaction where the group which is being added is an electrophile i.e. accepts a pair of electrons and nucleophilic addition is the addition reaction where the attacking group is a nucleophile i.e. it will donate a pair of electrons.
Similarly, electrophilic substitution is a reaction where the leaving group is substituted by an electrophile and nucleophilic substitution is a substitution reaction where the leaving group is replaced by the nucleophile by attacking on the positively charged atom to which the leaving group is attached.
To form cyanohydrin from ketone, we need a ketone and hydrogen cyanide.
The cyanide ion acts as a nucleophile which forms a bond with the electrophilic carbon centre of the ketone forming hydroxyacetonitrile which is commonly known as cyanohydrin. We can draw the mechanism of the reaction as-

The alkoxide anion is protonated which regenerates the cyanide ion to continue the reaction.
Here, as we can see a nucleophile attacks the electrophilic centre and as there is no substitution, it is just addition therefore, this reaction is a nucleophilic addition.
Therefore, the correct answer is option [B] Nucleophilic addition.
Note:
As hydrogen cyanide is an acid itself, it will need a base to take its proton which will leave behind a cyanide anion. The carbon centre of the cyanide anion will act as a nucleophile. If base is absent, the reaction proceeds at a very slow rate.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




