
The formula for ammonia is $N{H_3}$ .
A.True
B.False
Answer
581.1k+ views
Hint: The chemical formula of Ammonia is $N{H_3}$ . Ammonia consists of Hydrogen and Nitrogen. It is also known as trihydride nitrogen and also nitrogen trihydride. The density of Ammonia is 0.769 $\dfrac{{kg}}{{{m^3}}}$ at STP and it is lighter than air.
Complete step by step answer:
Ammonia is made of Nitrogen and Hydrogen. The electron configuration of Nitrogen (N) is \[1{s^2}2{s^2}2{p^3}\] . We can see that it needs 3 electrons to complete stability. The electronic configuration of Hydrogen (H) is \[1{s^2}\]
All the three hydrogen share their 1 electron with the Nitrogen and in this way, Nitrogen gains stability.
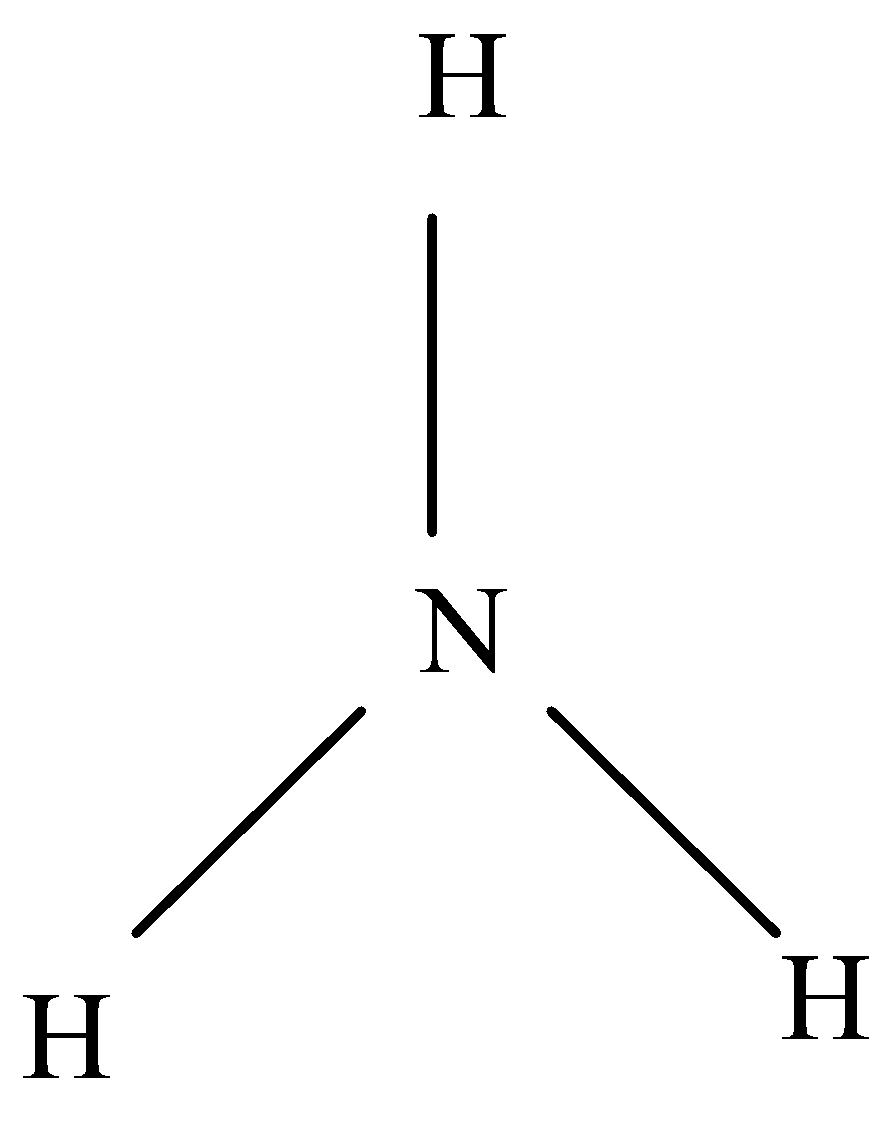
Ammonia is made in the laboratory by heating ammonium salt such as ammonium chloride with a strong alkali such as calcium hydroxide or sodium hydroxide. This process is known as Haber process, the combination of nitrogen and hydrogen under high pressure in the presence of a catalyst.
So, the formula of Ammonia is $N{H_3}$ .
Therefore, the correct answer is option (A).
Note: Ammonia behaves as a weak base because it combines with most acids to form salts. When Ammonia reacts with Hydrochloric acid, it converts into ammonium chloride. Ammonia is used in a household as the cleaner, it is mixed with water to clean the steel and glass, to increase the yield of crops, it is used as fertilizers, is used as a refrigerant, is used in the fermentation industry, is used in the fuels for rocket engine and is a preferred nitrogen containing nutrient for the growth of plants. Ammonia occurs naturally in soil, air or in vegetation. Ammonia is also a common pollutant and can be toxic and cause lower reproduction or even death.
Complete step by step answer:
Ammonia is made of Nitrogen and Hydrogen. The electron configuration of Nitrogen (N) is \[1{s^2}2{s^2}2{p^3}\] . We can see that it needs 3 electrons to complete stability. The electronic configuration of Hydrogen (H) is \[1{s^2}\]
All the three hydrogen share their 1 electron with the Nitrogen and in this way, Nitrogen gains stability.

Ammonia
Ammonia is made in the laboratory by heating ammonium salt such as ammonium chloride with a strong alkali such as calcium hydroxide or sodium hydroxide. This process is known as Haber process, the combination of nitrogen and hydrogen under high pressure in the presence of a catalyst.
So, the formula of Ammonia is $N{H_3}$ .
Therefore, the correct answer is option (A).
Note: Ammonia behaves as a weak base because it combines with most acids to form salts. When Ammonia reacts with Hydrochloric acid, it converts into ammonium chloride. Ammonia is used in a household as the cleaner, it is mixed with water to clean the steel and glass, to increase the yield of crops, it is used as fertilizers, is used as a refrigerant, is used in the fermentation industry, is used in the fuels for rocket engine and is a preferred nitrogen containing nutrient for the growth of plants. Ammonia occurs naturally in soil, air or in vegetation. Ammonia is also a common pollutant and can be toxic and cause lower reproduction or even death.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




