
The formula of chromyl chloride is:
A. CrCl
B. \[CrC{{l}_{3}}\]
C. CrOCl
D. \[Cr{{O}_{2}}C{{l}_{2}}\]
Answer
597.3k+ views
Hint: To answer the formula of chromyl chloride, we should first know about chromyl chloride. Chromyl chloride is made up of chromium with oxygen and chlorine. Here, chromium will have an oxidation state of 6.
Complete step by step solution:
We should first know about chromyl chloride. Chromyl chloride is a reddish-brown compound of chromium with oxygen and chlorine. This compound is extremely corrosive and will readily generate reddish brown fumes in air. It is also highly carcinogenic.
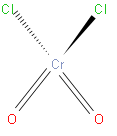
From the above structure we can say that it has one chromium atom and two chlorine atoms and two oxygen atoms. From this we can write the formula of chromyl chloride that is \[Cr{{O}_{2}}C{{l}_{2}}\].
So, from this we can say that option D is correct.
Addition information:
Chromyl chloride is used to determine the confirmation of chlorine ions. Let us take one sample salt with chlorine present in it. We should then heat it with potassium chromate (\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\]) and concentrated sulphuric acid (\[{{H}_{2}}S{{O}_{4}}\]). If chloride is present, chromyl chloride is formed and red fumes are given out.
We use this test for salts such as chlorides of mercury and silver. Chromyl chloride is often used as an oxidizing agent. The chromyl chloride can be used for the oxidation of toluene to benzaldehyde.
Note: We should be careful in handling of chromyl chloride. Exposure to chromyl chloride vapour irritates the respiratory system and severely irritates the eyes, and the liquid burns the skin and eyes.
Complete step by step solution:
We should first know about chromyl chloride. Chromyl chloride is a reddish-brown compound of chromium with oxygen and chlorine. This compound is extremely corrosive and will readily generate reddish brown fumes in air. It is also highly carcinogenic.

From the above structure we can say that it has one chromium atom and two chlorine atoms and two oxygen atoms. From this we can write the formula of chromyl chloride that is \[Cr{{O}_{2}}C{{l}_{2}}\].
So, from this we can say that option D is correct.
Addition information:
Chromyl chloride is used to determine the confirmation of chlorine ions. Let us take one sample salt with chlorine present in it. We should then heat it with potassium chromate (\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\]) and concentrated sulphuric acid (\[{{H}_{2}}S{{O}_{4}}\]). If chloride is present, chromyl chloride is formed and red fumes are given out.
We use this test for salts such as chlorides of mercury and silver. Chromyl chloride is often used as an oxidizing agent. The chromyl chloride can be used for the oxidation of toluene to benzaldehyde.
Note: We should be careful in handling of chromyl chloride. Exposure to chromyl chloride vapour irritates the respiratory system and severely irritates the eyes, and the liquid burns the skin and eyes.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




