
The formula of pernitric acid is:
A. \[{H_2}{N_2}{O_3}\]
B. \[HN{O_4}\]
C.\[{H_2}N{O_2}\]
D. \[{H_2}{N_2}{O_2}\]
Answer
586.2k+ views
Hint: Pernitric acid is a nitrogen oxoacid and a conjugate acid of peroxynitrite by forming rapidly during decomposition of peroxynitrite in a neutral environment. It is unstable yet a reservoir of nitrogen dioxide through the reversible radical reaction.
Complete step by step answer:
-Pernitric acid is also known as peroxynitric acid or peroxynitric acid. Its IUPAC name is hydroxy nitrate acid, it is not a formula for normal oxyacid. The oxidation state of nitrogen is +7 in it.
-Acids are divided into two groups. Binary acids have two elements and oxyacids have three elements out of which one is oxygen. Naming oxyacids is little difficult because these acids have a hydrogen, a non-metal and varying numbers of oxygen atoms. Let us take a reference point to name these oxyacids.
-The “ate” ions such as sulphate, nitrate etc. make the “ic” acids such as sulphuric acid, nitric acid, etc. using this point of reference we will name the acid having one more oxygen than the -ic acid as per-ic acid. The acid with one less oxygen than the -ic acid can be called as-ous acid.
-Now, we are given pernitric acid to determine its formula. It can be derived from the formula of nitric acid which is \[HN{O_3}\] . For pernitric acid, the acid must have one more oxygen than the nitric acid. Thus, its formula will be \[HN{O_4}\] .
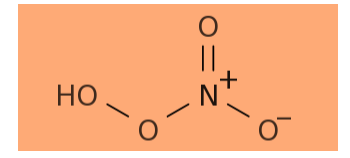
Hence, the correct option is (B).
Note:
For oxyacids of a given central atom, the acidity increases with the increase in the central element’s oxidation state or the number of oxygens bound to the central atom. This is due to the stability of the conjugate oxyanion. The main determining factor for their relative strength is the electronegativity of the central atom.
Complete step by step answer:
-Pernitric acid is also known as peroxynitric acid or peroxynitric acid. Its IUPAC name is hydroxy nitrate acid, it is not a formula for normal oxyacid. The oxidation state of nitrogen is +7 in it.
-Acids are divided into two groups. Binary acids have two elements and oxyacids have three elements out of which one is oxygen. Naming oxyacids is little difficult because these acids have a hydrogen, a non-metal and varying numbers of oxygen atoms. Let us take a reference point to name these oxyacids.
-The “ate” ions such as sulphate, nitrate etc. make the “ic” acids such as sulphuric acid, nitric acid, etc. using this point of reference we will name the acid having one more oxygen than the -ic acid as per-ic acid. The acid with one less oxygen than the -ic acid can be called as-ous acid.
-Now, we are given pernitric acid to determine its formula. It can be derived from the formula of nitric acid which is \[HN{O_3}\] . For pernitric acid, the acid must have one more oxygen than the nitric acid. Thus, its formula will be \[HN{O_4}\] .

Hence, the correct option is (B).
Note:
For oxyacids of a given central atom, the acidity increases with the increase in the central element’s oxidation state or the number of oxygens bound to the central atom. This is due to the stability of the conjugate oxyanion. The main determining factor for their relative strength is the electronegativity of the central atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




