
The formula of quinine is
A.\[{C_{20}}{H_{24}}{N_2}{O_2}\]
B.\[{C_{19}}{H_{22}}{N_2}O\]
C.\[{C_{19}}{H_{22}}{N_2}{O_2}\]
D.\[{C_{19}}{H_{24}}{N_2}{O_2}\]
Answer
519.6k+ views
Hint: Quinine is an antimalarial and anti-babesiosis drug. When artesunate is not available, this requires the treatment of malaria caused by Plasmodium falciparum, which is immune to chloroquine. Although quinine is often used to treat restless legs syndrome, it is not prescribed because of the possibility of severe side effects. It may be consumed orally or intravenously. In certain parts of the country, malaria susceptibility to quinine exists. Quinine is also the bitterness-inducing component in tonic water.
Complete answer:
Quinine was extracted for the first time in 1820 from the bark of a Peruvian cinchona tree. Malaria has been treated with bark extracts since at least 1632, and it was brought to Spain by Jesuit missionaries from the New World as early as 1636. It is classified as an essential medicine by the World Health Organization. Quinine's UV absorption peaks at 350 nanometers (in UVA). About 460 nm (bright blue/cyan hue), fluorescent emission peaks. Quinine fluoresces brightly in 0.1 M sulfuric acid solution (quantum yield 0.58).
The enzyme strictosidine synthase catalyses a stereoselective Pictet–Spengler reaction between tryptamine and secologanin to produce strictosidine in the first step of quinine biosynthesis. Strictosidine can be converted to an aldehyde with the right modifications. Corynantheal is produced by hydrolysis and decarboxylation, which removes one carbon from the iridoid component. The tryptamine side chain was then cleaved adjacent to the nitrogen, which was then bound to the acetaldehyde function to produce cinchonaminal. The indole heterocyclic ring's ring opening may lead to new amine and keto functions. This amine will then be combined with the aldehyde generated during the tryptamine side-chain cleavage to form cinchonidinone, a new quinoline heterocycle. The final step is hydroxylation and methylation, which produces quinine.
The molecular formula of Quinine is \[{C_{20}}{H_{24}}{N_2}{O_2}\]
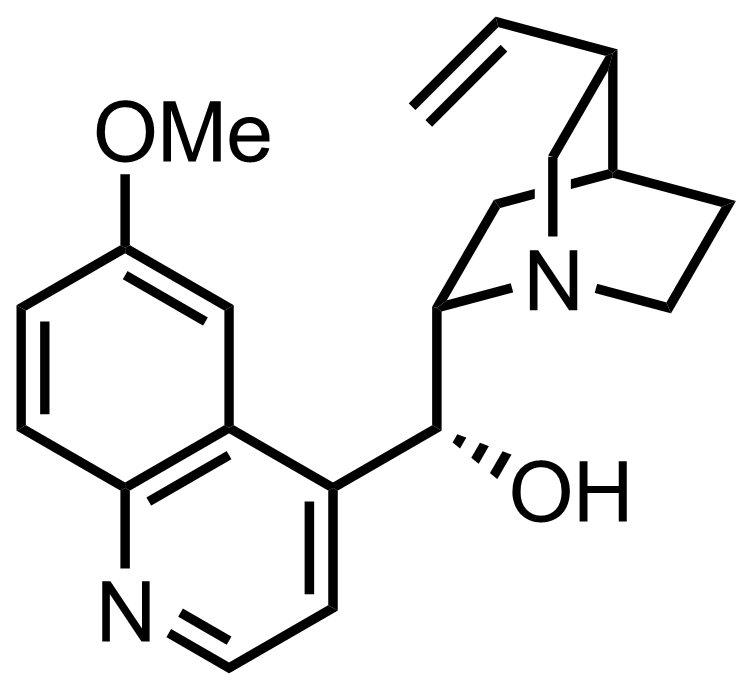
Note:
Quinine is used because it inhibits Plasmodium falciparum's ability to degrade and metabolize haemoglobin, making it toxic to the parasite. The exact mechanism of action of quinine, as that of other quinoline antimalarial medications, is unknown, though in vitro experiments show that it inhibits nucleic acid and protein synthesis, as well as glycolysis, in P. falciparum. The most widely held theory of action is based on chloroquine, a well-studied and closely related quinoline compound. Inhibition of hemozoin biocrystallization in the heme detoxification pathway, which promotes the aggregation of cytotoxic heme, is part of this model.
Complete answer:
Quinine was extracted for the first time in 1820 from the bark of a Peruvian cinchona tree. Malaria has been treated with bark extracts since at least 1632, and it was brought to Spain by Jesuit missionaries from the New World as early as 1636. It is classified as an essential medicine by the World Health Organization. Quinine's UV absorption peaks at 350 nanometers (in UVA). About 460 nm (bright blue/cyan hue), fluorescent emission peaks. Quinine fluoresces brightly in 0.1 M sulfuric acid solution (quantum yield 0.58).
The enzyme strictosidine synthase catalyses a stereoselective Pictet–Spengler reaction between tryptamine and secologanin to produce strictosidine in the first step of quinine biosynthesis. Strictosidine can be converted to an aldehyde with the right modifications. Corynantheal is produced by hydrolysis and decarboxylation, which removes one carbon from the iridoid component. The tryptamine side chain was then cleaved adjacent to the nitrogen, which was then bound to the acetaldehyde function to produce cinchonaminal. The indole heterocyclic ring's ring opening may lead to new amine and keto functions. This amine will then be combined with the aldehyde generated during the tryptamine side-chain cleavage to form cinchonidinone, a new quinoline heterocycle. The final step is hydroxylation and methylation, which produces quinine.
The molecular formula of Quinine is \[{C_{20}}{H_{24}}{N_2}{O_2}\]

Note:
Quinine is used because it inhibits Plasmodium falciparum's ability to degrade and metabolize haemoglobin, making it toxic to the parasite. The exact mechanism of action of quinine, as that of other quinoline antimalarial medications, is unknown, though in vitro experiments show that it inhibits nucleic acid and protein synthesis, as well as glycolysis, in P. falciparum. The most widely held theory of action is based on chloroquine, a well-studied and closely related quinoline compound. Inhibition of hemozoin biocrystallization in the heme detoxification pathway, which promotes the aggregation of cytotoxic heme, is part of this model.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




