
The general molecular formula for disaccharide is:
A) $\text{ }{{\text{C}}_{\text{12}}}{{\text{H}}_{\text{22}}}{{\text{O}}_{\text{11}}}\text{ }$
B) $\text{ }{{\text{C}}_{\text{10}}}{{\text{H}}_{\text{20}}}{{\text{O}}_{\text{10}}}\text{ }$
C) $\text{ }{{\text{C}}_{\text{12}}}{{\text{H}}_{\text{20}}}{{\text{O}}_{\text{10}}}\text{ }$
D) $\text{ }{{\text{C}}_{\text{12}}}{{\text{H}}_{\text{22}}}{{\text{O}}_{\text{10}}}\text{ }$
Answer
582.9k+ views
Hint: The disaccharides are the carbohydrates which are composed of the two same or different monosaccharides. The common found disaccharide are as sucrose: made of glucose and fructose, maltose: made of two glucose molecules and lactose: made of glucose and galactose. The monosaccharides are linked together by the glycosidic linkage.
Complete step by step answer:
Disaccharides are the carbohydrates which on hydrolysis gives two same or different monosaccharides. There general formula is$\text{ }{{\text{C}}_{\text{12}}}{{\text{H}}_{\text{22}}}{{\text{O}}_{\text{11}}}\text{ }$. The important members belonging to disaccharides are sucrose, maltose, and lactose. On hydrolysis with dilute acid or enzyme, these give the following two molecules of monosaccharides.
$\text{ }\begin{matrix}
\text{Sucrose} & \xrightarrow[\text{or Invertase}]{{{\text{H}}^{\text{+}}}} & \text{Glucose} & \text{+} & \text{Fructose} \\
\text{Maltose} & \xrightarrow[\text{or Maltase}]{{{\text{H}}^{\text{+}}}} & \text{Glucose} & \text{+} & \text{Glucose} \\
\text{Lactose} & \xrightarrow[\text{or Lactase}]{{{\text{H}}^{\text{+}}}} & \text{Glucose} & \text{+} & \text{Galactose} \\
\end{matrix}\text{ }$
The disaccharides are made up of two molecules of monosaccharides linked to each other by the condensation reaction. The linking is formed just as hemiacetals react with alcohols to form acetal with the elimination of water molecules.
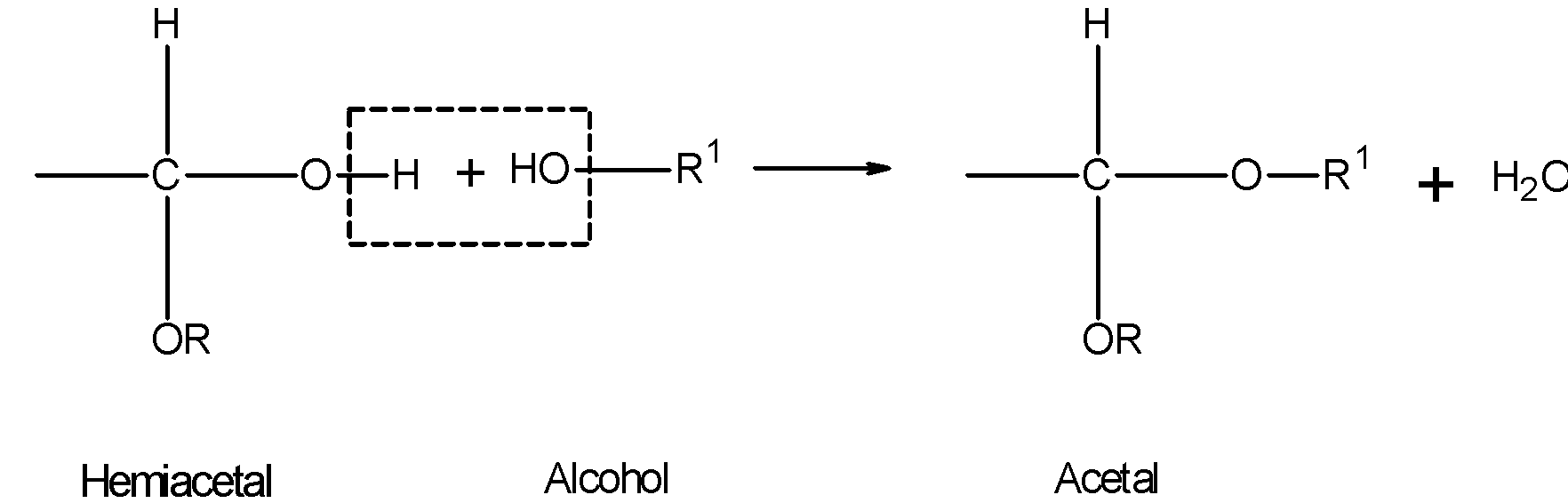
Similarly hydroxyl groups of the hemiacetals of two monosaccharides units condense to form disaccharides. The two monosaccharides units are linked to each other by a bond called the glycosidic linkage.
Let's consider an example of a common disaccharide
A) Sucrose.
It is the most common disaccharide it is widely distributed in plants, particularly sugar cane and sugar beet. It is manufactured from cane or sugar beet. It is very soluble in the water and its aqueous solution is dextrorotatory having specific rotation equal to\[\text{ }+{{66.5}^{0}}\text{ }\]. On hydrolysis with dilute acid or the enzyme invertase, cane sugar (sucrose) gives an equimolar mixture of D-fructose and D-glucose. The reaction is as follows,
$\text{ }\begin{matrix}
{{\text{C}}_{\text{12}}}{{\text{H}}_{\text{22}}}{{\text{O}}_{\text{11}}} & \text{+} & {{\text{H}}_{\text{2}}}\text{O} & \xrightarrow[\text{Or Invertase}]{\text{H+}} & {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}} & \text{+} & {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}} \\
\text{(Sucrose)} & {} & {} & {} & \text{(Glucose)} & {} & \text{(Fructose)} \\
\end{matrix}\text{ }$
B) Maltose:
It is known as malt sugar. It is a principal disaccharide obtained by the partial hydrolysis of starch by diastase, an enzyme present in malt (sprouted barley seeds)
Reaction
On hydrolysis, the maltose gives two moles of $\text{ }\alpha -\text{D}-\text{ }$ glucose. It is a reducing sugar.
$\text{ }\begin{matrix}
{{\text{C}}_{\text{12}}}{{\text{H}}_{\text{22}}}{{\text{O}}_{\text{11}}} & \text{+} & {{\text{H}}_{\text{2}}}\text{O} & \xrightarrow[\text{Or Maltase}]{\text{H+}} & {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}} & \text{+} & {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}} \\
\text{(Maltose)} & {} & {} & {} & \text{(Glucose)} & {} & \text{(Glucose)} \\
\end{matrix}\text{ }$
C) Lactose:
It is a disaccharide.it occurs in the milk and therefore, it is also called the milk sugar. The lactose has the general formula$\text{ }{{\text{C}}_{\text{12}}}{{\text{H}}_{\text{22}}}{{\text{O}}_{\text{11}}}\text{ }$. Lactose on hydrolysis with the dilute acid gives the equimolar mixture of $\text{ }\beta -\text{D}-\text{ }$glucose and $\text{ }\beta -\text{D}-\text{ }$galactose. It is reducing sugar. Therefore, it is composed of $\text{ }\beta -\text{D}-\text{ }$glucose and $\text{ }\beta -\text{D}-\text{ }$galactose units. These units are held together by the glycosidic linkage.
$\begin{matrix}
{{\text{C}}_{\text{12}}}{{\text{H}}_{\text{22}}}{{\text{O}}_{\text{11}}} & \text{+} & {{\text{H}}_{\text{2}}}\text{O} & \xrightarrow[\text{Or lactase}]{\text{H+}} & {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}} & \text{+} & {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}} \\
\text{(Lactose)} & {} & {} & {} & \text{(Glucose)} & {} & \text{(Galactose)} \\
\end{matrix}\text{ }$
Here, we observe that the disaccharides such as the sucrose, maltose and lactose have the general molecular formula of$\text{ }{{\text{C}}_{\text{12}}}{{\text{H}}_{\text{22}}}{{\text{O}}_{\text{11}}}\text{ }$.
So, the correct answer is “Option A”.
Note: Note that, the sucrose is a disaccharide but the bacteria in the mouth have an enzyme which converts the sucrose into the polysaccharide called as a dextran. About $\text{ 10 }{\scriptstyle{}^{0}/{}_{0}}\text{ }$ of the dental plaque is composed of dextran. That is why dentists caution you not to eat candy.
Complete step by step answer:
Disaccharides are the carbohydrates which on hydrolysis gives two same or different monosaccharides. There general formula is$\text{ }{{\text{C}}_{\text{12}}}{{\text{H}}_{\text{22}}}{{\text{O}}_{\text{11}}}\text{ }$. The important members belonging to disaccharides are sucrose, maltose, and lactose. On hydrolysis with dilute acid or enzyme, these give the following two molecules of monosaccharides.
$\text{ }\begin{matrix}
\text{Sucrose} & \xrightarrow[\text{or Invertase}]{{{\text{H}}^{\text{+}}}} & \text{Glucose} & \text{+} & \text{Fructose} \\
\text{Maltose} & \xrightarrow[\text{or Maltase}]{{{\text{H}}^{\text{+}}}} & \text{Glucose} & \text{+} & \text{Glucose} \\
\text{Lactose} & \xrightarrow[\text{or Lactase}]{{{\text{H}}^{\text{+}}}} & \text{Glucose} & \text{+} & \text{Galactose} \\
\end{matrix}\text{ }$
The disaccharides are made up of two molecules of monosaccharides linked to each other by the condensation reaction. The linking is formed just as hemiacetals react with alcohols to form acetal with the elimination of water molecules.

Similarly hydroxyl groups of the hemiacetals of two monosaccharides units condense to form disaccharides. The two monosaccharides units are linked to each other by a bond called the glycosidic linkage.
Let's consider an example of a common disaccharide
A) Sucrose.
It is the most common disaccharide it is widely distributed in plants, particularly sugar cane and sugar beet. It is manufactured from cane or sugar beet. It is very soluble in the water and its aqueous solution is dextrorotatory having specific rotation equal to\[\text{ }+{{66.5}^{0}}\text{ }\]. On hydrolysis with dilute acid or the enzyme invertase, cane sugar (sucrose) gives an equimolar mixture of D-fructose and D-glucose. The reaction is as follows,
$\text{ }\begin{matrix}
{{\text{C}}_{\text{12}}}{{\text{H}}_{\text{22}}}{{\text{O}}_{\text{11}}} & \text{+} & {{\text{H}}_{\text{2}}}\text{O} & \xrightarrow[\text{Or Invertase}]{\text{H+}} & {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}} & \text{+} & {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}} \\
\text{(Sucrose)} & {} & {} & {} & \text{(Glucose)} & {} & \text{(Fructose)} \\
\end{matrix}\text{ }$
B) Maltose:
It is known as malt sugar. It is a principal disaccharide obtained by the partial hydrolysis of starch by diastase, an enzyme present in malt (sprouted barley seeds)
Reaction
On hydrolysis, the maltose gives two moles of $\text{ }\alpha -\text{D}-\text{ }$ glucose. It is a reducing sugar.
$\text{ }\begin{matrix}
{{\text{C}}_{\text{12}}}{{\text{H}}_{\text{22}}}{{\text{O}}_{\text{11}}} & \text{+} & {{\text{H}}_{\text{2}}}\text{O} & \xrightarrow[\text{Or Maltase}]{\text{H+}} & {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}} & \text{+} & {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}} \\
\text{(Maltose)} & {} & {} & {} & \text{(Glucose)} & {} & \text{(Glucose)} \\
\end{matrix}\text{ }$
C) Lactose:
It is a disaccharide.it occurs in the milk and therefore, it is also called the milk sugar. The lactose has the general formula$\text{ }{{\text{C}}_{\text{12}}}{{\text{H}}_{\text{22}}}{{\text{O}}_{\text{11}}}\text{ }$. Lactose on hydrolysis with the dilute acid gives the equimolar mixture of $\text{ }\beta -\text{D}-\text{ }$glucose and $\text{ }\beta -\text{D}-\text{ }$galactose. It is reducing sugar. Therefore, it is composed of $\text{ }\beta -\text{D}-\text{ }$glucose and $\text{ }\beta -\text{D}-\text{ }$galactose units. These units are held together by the glycosidic linkage.
$\begin{matrix}
{{\text{C}}_{\text{12}}}{{\text{H}}_{\text{22}}}{{\text{O}}_{\text{11}}} & \text{+} & {{\text{H}}_{\text{2}}}\text{O} & \xrightarrow[\text{Or lactase}]{\text{H+}} & {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}} & \text{+} & {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}} \\
\text{(Lactose)} & {} & {} & {} & \text{(Glucose)} & {} & \text{(Galactose)} \\
\end{matrix}\text{ }$
Here, we observe that the disaccharides such as the sucrose, maltose and lactose have the general molecular formula of$\text{ }{{\text{C}}_{\text{12}}}{{\text{H}}_{\text{22}}}{{\text{O}}_{\text{11}}}\text{ }$.
So, the correct answer is “Option A”.
Note: Note that, the sucrose is a disaccharide but the bacteria in the mouth have an enzyme which converts the sucrose into the polysaccharide called as a dextran. About $\text{ 10 }{\scriptstyle{}^{0}/{}_{0}}\text{ }$ of the dental plaque is composed of dextran. That is why dentists caution you not to eat candy.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




