
The geometry of the ozone molecule is:
A.Linear
B.V-shaped
C.Any of these
D.None of these
Answer
577.8k+ views
Hint: To answer this question, you should recall the concept of the effect of lone pairs on the structure of any molecule. The molecule of Ozone has three oxygen atoms and the chemical symbol of \[{O_3}\] . The central atom has 3 bond pairs and 1 lone pair.
Complete step by step answer:
In Ozone, there are six valence electrons for each molecule of Oxygen. Here as there are three oxygen molecules, the total number of valence electrons is \[6 \times 3 = {\text{ }}18\] . The central atom has one lone pair of electrons and is stable due to the eight electrons in its outermost orbit.
In the structure of ozone, there are 2 sigma bonds, each sigma bond between the two oxygen atoms and there is one pi bond between the central oxygen atom and left most oxygen atom. Electrons of central oxygen will repel the electron cloud of the two oxygen atoms on each end. This will result in the end oxygen groups being pushed down giving the \[{O_3}\] molecule a bent molecular geometry or V shape.
The structure can be drawn as:
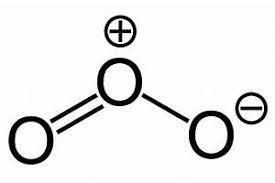
Hence the correct answer to this question is option B.
Note:
Make sure you remember the concept of the polarity of molecules and its dependence on its molecular geometry. Here, the Ozone molecule is bent due to its valence electrons. All three Oxygen molecules are not linear due to their \[s{p^2}\] hybridization. As the molecules are not in linear geometry there, dipole interactions are not nullified, and there is net dipole on its molecule. Thus, there is polarity in Ozone, and it can be said that Ozone is polar.
Complete step by step answer:
In Ozone, there are six valence electrons for each molecule of Oxygen. Here as there are three oxygen molecules, the total number of valence electrons is \[6 \times 3 = {\text{ }}18\] . The central atom has one lone pair of electrons and is stable due to the eight electrons in its outermost orbit.
In the structure of ozone, there are 2 sigma bonds, each sigma bond between the two oxygen atoms and there is one pi bond between the central oxygen atom and left most oxygen atom. Electrons of central oxygen will repel the electron cloud of the two oxygen atoms on each end. This will result in the end oxygen groups being pushed down giving the \[{O_3}\] molecule a bent molecular geometry or V shape.
The structure can be drawn as:

Hence the correct answer to this question is option B.
Note:
Make sure you remember the concept of the polarity of molecules and its dependence on its molecular geometry. Here, the Ozone molecule is bent due to its valence electrons. All three Oxygen molecules are not linear due to their \[s{p^2}\] hybridization. As the molecules are not in linear geometry there, dipole interactions are not nullified, and there is net dipole on its molecule. Thus, there is polarity in Ozone, and it can be said that Ozone is polar.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




