
The good method for converting benzene into propyl benzene is:
[A] ${{C}_{6}}{{H}_{6}}+C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}Cl+Anhyd.AlC{{l}_{3}}$
[B] ${{C}_{6}}{{H}_{6}}+C{{H}_{3}}C{{H}_{3}}C{{H}_{2}}COCl+Anhyd.AlC{{l}_{3}}\text{ and then treatment with Zn/Hg/HCl}$
[C] ${{C}_{6}}{{H}_{6}}+C{{H}_{3}}C{{H}_{3}}C{{H}_{2}}COCl+Anhyd.AlC{{l}_{3}}\text{ and then treatment with }{{\text{H}}_{2}}Ni$
[D] ${{C}_{6}}{{H}_{6}}+Anhyd.AlC{{l}_{3}}\text{ +cyclopropane}$
Answer
524.9k+ views
Hint: To convert benzene into propyl benzene we use a method named as Friedel Crafts Alkylation. The method involves alkylation of an aromatic ring with an alkyl halide using a strong Lewis acid like aluminium chloride or ferric chloride.
Complete step by step answer:
We have to convert benzene which is an aromatic ring into propyl benzene which includes an alkyl chain added to the benzene ring.
If we want to add a carbon substituent to an aromatic nucleophile, we need reactive carbon electrophiles which are known as carbocations.
In the Friedel-Crafts alkylation, we treat benzene with tertiary alkyl chloride and a Lewis acid like $AlC{{l}_{3}}$ . The Lewis acid removes the chlorine atom to form a tertiary butyl chloride and then releases the tertiary butyl cation for the alkylation reaction.
We generally do not have to worry about the base that removes the proton from the intermediate. Anything such as water, chloride or other counter ions of strong acids can get this job done easily and thus does not require any exact reagent. Here, it is important that the alkyl group can form a cation otherwise the reaction does not proceed forward very well.
Here, among the given options, two of them can give us the required product conveniently. Let us discuss them one by one.
In the first option, the –R group is not appropriate for alkylation. It gives two products where the propyl benzene is a minor product. Thus it is inconvenient and cannot be used.
In the second option, we have a –R group which can form a stable carbocation and attach to the benzene ring and give us a ketone attached to the ring and the reagents Zn, Hg and HCl is used which reduces the ketone and gives us hydrocarbon thus propyl benzene. We can write the reaction as-
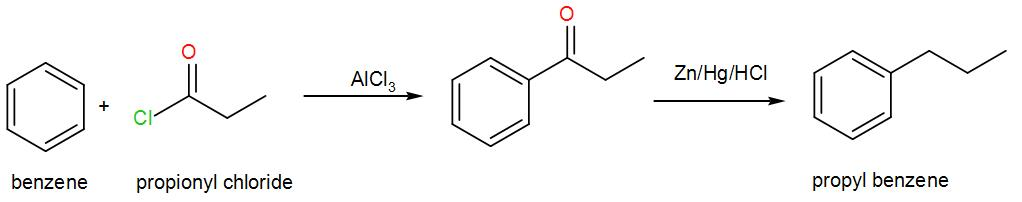
In the third option, we have hydrogen and nickel, also known as Raney nickel which is a strong reducing agent but it reduces ketones and aldehydes to alcohol therefore, it cannot be used here.
In the last option, we have cyclopropane with anhydrous aluminium chloride which can be used for the alkylation. We can write the reaction as-
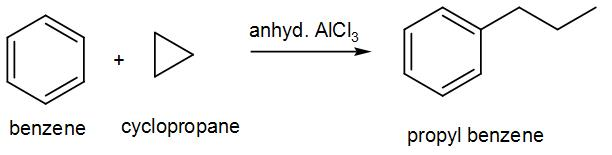
We can see from the above discussion that there are two possible processes.
${{C}_{6}}{{H}_{6}}+C{{H}_{3}}C{{H}_{3}}C{{H}_{2}}COCl+Anhyd.AlC{{l}_{3}}\text{ and then treatment with Zn/Hg/HCl}$ and [D] ${{C}_{6}}{{H}_{6}}+Anhyd.AlC{{l}_{3}}\text{ +cyclopropane}$
So, the correct answers are “Option B and D”.
Note: Similar to the Friedel-Crafts Alkylation, there is another method named Friedel-Crafts Acylation which gives us aryl ketones. The acylation is a better option compared to alkylation because it does not require any particular structural feature in the acyl chloride. Here, -R can be anything unlike alkylation reactions where –R should form a stable cation. Also acylation stops after one reaction but alkylation gives a mixture of products.
Complete step by step answer:
We have to convert benzene which is an aromatic ring into propyl benzene which includes an alkyl chain added to the benzene ring.
If we want to add a carbon substituent to an aromatic nucleophile, we need reactive carbon electrophiles which are known as carbocations.
In the Friedel-Crafts alkylation, we treat benzene with tertiary alkyl chloride and a Lewis acid like $AlC{{l}_{3}}$ . The Lewis acid removes the chlorine atom to form a tertiary butyl chloride and then releases the tertiary butyl cation for the alkylation reaction.
We generally do not have to worry about the base that removes the proton from the intermediate. Anything such as water, chloride or other counter ions of strong acids can get this job done easily and thus does not require any exact reagent. Here, it is important that the alkyl group can form a cation otherwise the reaction does not proceed forward very well.
Here, among the given options, two of them can give us the required product conveniently. Let us discuss them one by one.
In the first option, the –R group is not appropriate for alkylation. It gives two products where the propyl benzene is a minor product. Thus it is inconvenient and cannot be used.
In the second option, we have a –R group which can form a stable carbocation and attach to the benzene ring and give us a ketone attached to the ring and the reagents Zn, Hg and HCl is used which reduces the ketone and gives us hydrocarbon thus propyl benzene. We can write the reaction as-

In the third option, we have hydrogen and nickel, also known as Raney nickel which is a strong reducing agent but it reduces ketones and aldehydes to alcohol therefore, it cannot be used here.
In the last option, we have cyclopropane with anhydrous aluminium chloride which can be used for the alkylation. We can write the reaction as-

We can see from the above discussion that there are two possible processes.
${{C}_{6}}{{H}_{6}}+C{{H}_{3}}C{{H}_{3}}C{{H}_{2}}COCl+Anhyd.AlC{{l}_{3}}\text{ and then treatment with Zn/Hg/HCl}$ and [D] ${{C}_{6}}{{H}_{6}}+Anhyd.AlC{{l}_{3}}\text{ +cyclopropane}$
So, the correct answers are “Option B and D”.
Note: Similar to the Friedel-Crafts Alkylation, there is another method named Friedel-Crafts Acylation which gives us aryl ketones. The acylation is a better option compared to alkylation because it does not require any particular structural feature in the acyl chloride. Here, -R can be anything unlike alkylation reactions where –R should form a stable cation. Also acylation stops after one reaction but alkylation gives a mixture of products.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




