
The graphical representation of vapour pressure of two component systems as a function of composition is given alongside. By graphic inspection, answer the following question:
Name the type of deviation shown by this system from Raoult’s Law.
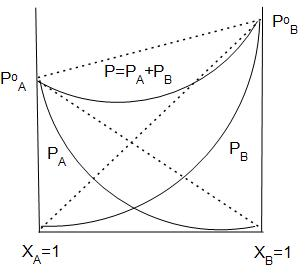

Answer
566.7k+ views
Hint: To solve this question, you must recall Raoult's law. Raoult’s Law states that in a solution, the partial vapour pressure of each volatile compound is directly proportional to its mole fraction.
Complete step by step answer:
Deviation from Raoult's law may either be positive deviation or a negative deviation.
Positive deviation is said to be shown in a mixture if the vapour pressure of the mixture is found to be higher than that expected on the basis of the Raoult’s law. It is observed when the cohesive forces between the similar molecules of each liquid are greater than the adhesive forces between the molecules of different liquids.
That is A-A and B-B interaction > A-B interaction
Negative deviation is said to be shown in a mixture if the vapour pressure of the mixture is found to be lesser than that expected on the basis of the Raoult’s law. It is observed when the cohesive forces between the similar molecules of each liquid are weaker than the adhesive forces between the molecules of different liquids.
That is A-A and B-B interaction > A-B interaction.
In the graph we can see clearly that the observed vapour pressure is lesser than the expected vapour pressure.
Thus, the system shows negative deviation from Raoult’s law.
Note:
Raoult’s law is given for an ideal mixture. A solution that shows a deviation from Raoult's law over the entire range of various compositions of the mixture, the solution is said to be a non- ideal or real solution. In a real solution, the pair of liquids that constitute the mixture do not have a uniformity throughout attractive forces, which may be either adhesive or cohesive. Thus, they show a deviation from Raoult's Law.
Complete step by step answer:
Deviation from Raoult's law may either be positive deviation or a negative deviation.
Positive deviation is said to be shown in a mixture if the vapour pressure of the mixture is found to be higher than that expected on the basis of the Raoult’s law. It is observed when the cohesive forces between the similar molecules of each liquid are greater than the adhesive forces between the molecules of different liquids.
That is A-A and B-B interaction > A-B interaction
Negative deviation is said to be shown in a mixture if the vapour pressure of the mixture is found to be lesser than that expected on the basis of the Raoult’s law. It is observed when the cohesive forces between the similar molecules of each liquid are weaker than the adhesive forces between the molecules of different liquids.
That is A-A and B-B interaction > A-B interaction.
In the graph we can see clearly that the observed vapour pressure is lesser than the expected vapour pressure.
Thus, the system shows negative deviation from Raoult’s law.
Note:
Raoult’s law is given for an ideal mixture. A solution that shows a deviation from Raoult's law over the entire range of various compositions of the mixture, the solution is said to be a non- ideal or real solution. In a real solution, the pair of liquids that constitute the mixture do not have a uniformity throughout attractive forces, which may be either adhesive or cohesive. Thus, they show a deviation from Raoult's Law.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




