
The groups of heterocyclic compounds is :
A.phenol, furan
B.furan, thiophene
C.thiophene, phenol
D.furan, aniline
Answer
233.1k+ views
Hint:Heterocyclic compounds are cyclic structures containing atoms of at least two different elements in its ring structure.
Complete step by step answer:
Cyclic compounds are a category of organic/inorganic compounds in which some or all constituent elements or constituent compounds are arranged in a ring like structure. Although most of these cyclic compounds are organic in nature. If all the atoms that form the ring are carbon, the compound is said to be Carbocyclic; if not, the compound is called heterocyclic. Carbocyclic compounds are more commonly known as homocyclic compounds. Also, a cyclic compound which behaves chemically just as an open-chain aliphatic compound is said to be alicyclic.
In the case of hetero cyclic compounds, as the name suggests, one of the ring members is different in comparison to the remaining ring members. The number of hetero atoms can be more than one as well. Usually, heterocyclic compounds with 5 or 6 member rings are more stable than other possible configurations.
Now, coming back to the question, in order to properly determine the heterocyclic pair out of the given options, we must first understand the molecular structure of these compounds.
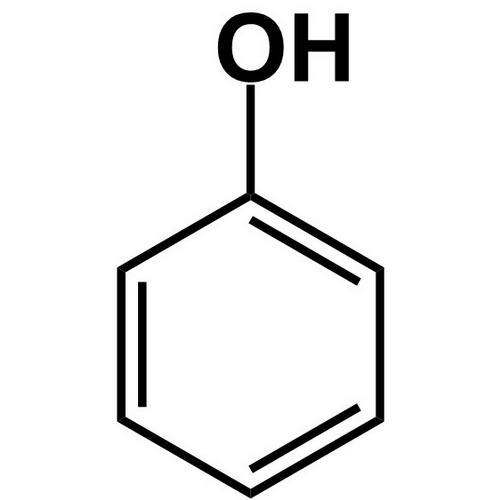
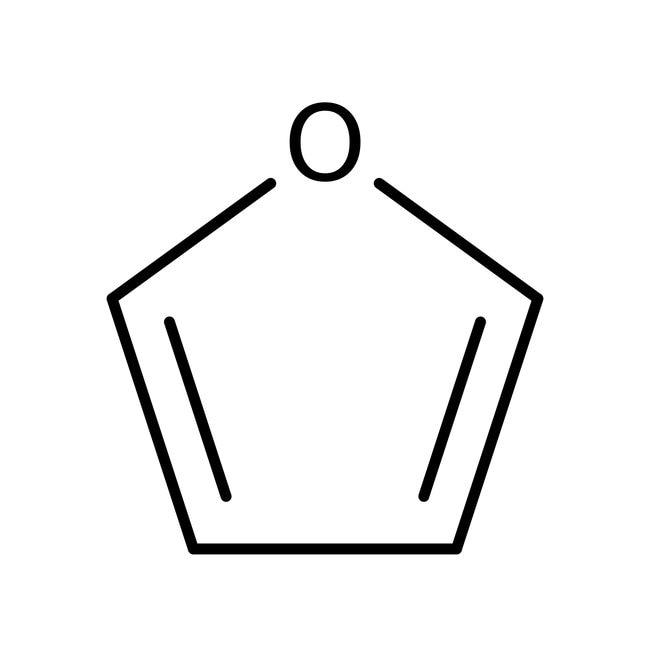
1. Phenol 2. furan
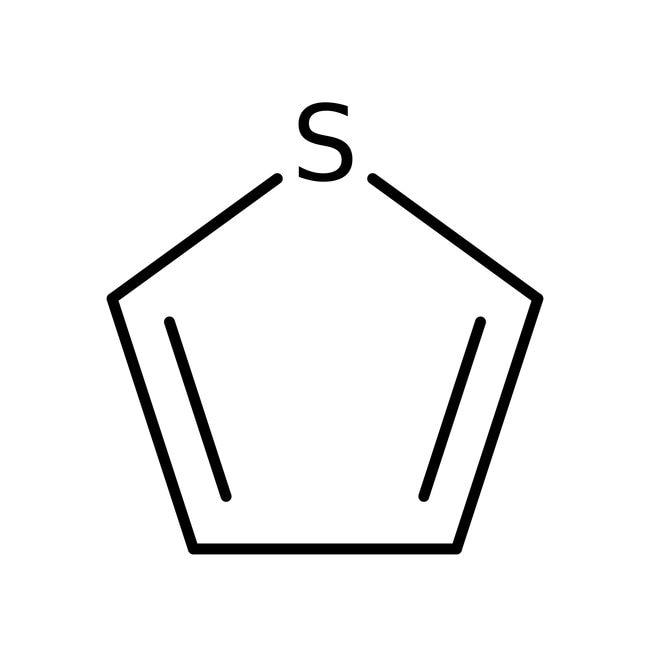
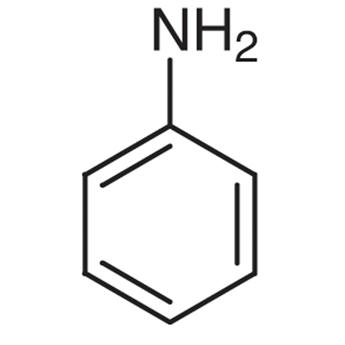
3.thiophene 4. Aniline
From the molecular structures of the given compounds represented above, we can determine the following things:
1.Phenol is NOT a heterocyclic compound because all the members of the ring structure are carbon.
2.Furan is a heterocyclic compound because it contains a hetero atom, i.e. oxygen
3.Thiophene is also a heterocyclic compound because it contains a hetero atom, i.e. sulphur
4.Aniline is NOT a heterocyclic compound because all the members of the ring structure are carbon.
Hence, Option B is the correct answer.
Note: The 3- and 4-member ring structure compounds are not stable and hence cannot be considered heterocyclic in nature.
Complete step by step answer:
Cyclic compounds are a category of organic/inorganic compounds in which some or all constituent elements or constituent compounds are arranged in a ring like structure. Although most of these cyclic compounds are organic in nature. If all the atoms that form the ring are carbon, the compound is said to be Carbocyclic; if not, the compound is called heterocyclic. Carbocyclic compounds are more commonly known as homocyclic compounds. Also, a cyclic compound which behaves chemically just as an open-chain aliphatic compound is said to be alicyclic.
In the case of hetero cyclic compounds, as the name suggests, one of the ring members is different in comparison to the remaining ring members. The number of hetero atoms can be more than one as well. Usually, heterocyclic compounds with 5 or 6 member rings are more stable than other possible configurations.
Now, coming back to the question, in order to properly determine the heterocyclic pair out of the given options, we must first understand the molecular structure of these compounds.


1. Phenol 2. furan


3.thiophene 4. Aniline
From the molecular structures of the given compounds represented above, we can determine the following things:
1.Phenol is NOT a heterocyclic compound because all the members of the ring structure are carbon.
2.Furan is a heterocyclic compound because it contains a hetero atom, i.e. oxygen
3.Thiophene is also a heterocyclic compound because it contains a hetero atom, i.e. sulphur
4.Aniline is NOT a heterocyclic compound because all the members of the ring structure are carbon.
Hence, Option B is the correct answer.
Note: The 3- and 4-member ring structure compounds are not stable and hence cannot be considered heterocyclic in nature.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




