
The $H-C-H$ bond angle in $C{{H}_{4}}$ is ${{109.5}^{\circ }}$, due to the presence lone pair, the $H-O-H$ angle in ${{H}_{2}}O$ will:
(a)- Remain same
(b)- Increases
(c)- Decreases
(d)- Becomes ${{180}^{\circ }}$
Answer
588.6k+ views
Hint: If the molecule does not have any lone pair then the structure of the molecule is symmetric. Due to the symmetric structure, the bond angle remains the same. If the molecule has lone pairs, the bond pairs will experience repulsion hence the bonds will come closer.
Complete step by step answer:
The bond angle of a molecule tells the direction of the bonds.
When the molecule has a symmetric structure, i.e., they have only bond pairs. The bond angle can easily be determined. According to the hybridization of the molecule, the bond angle of the molecule can be predicted. If the molecule has $sp$ hybridization the bond angle is ${{180}^{\circ }}$, if the molecule has $s{{p}^{2}}$ hybridization, the bond angle is ${{120}^{\circ }}$, if the molecule has $s{{p}^{3}}$ hybridization the bond angle is ${{109.5}^{\circ }}$, etc.
The $C{{H}_{4}}$ molecule has a symmetric structure because it has four bond pairs. The hybridization of carbon in $C{{H}_{4}}$ is $s{{p}^{3}}$. The bond angle between the atoms is ${{109.5}^{\circ }}$. The structure of $C{{H}_{4}}$ is given below:
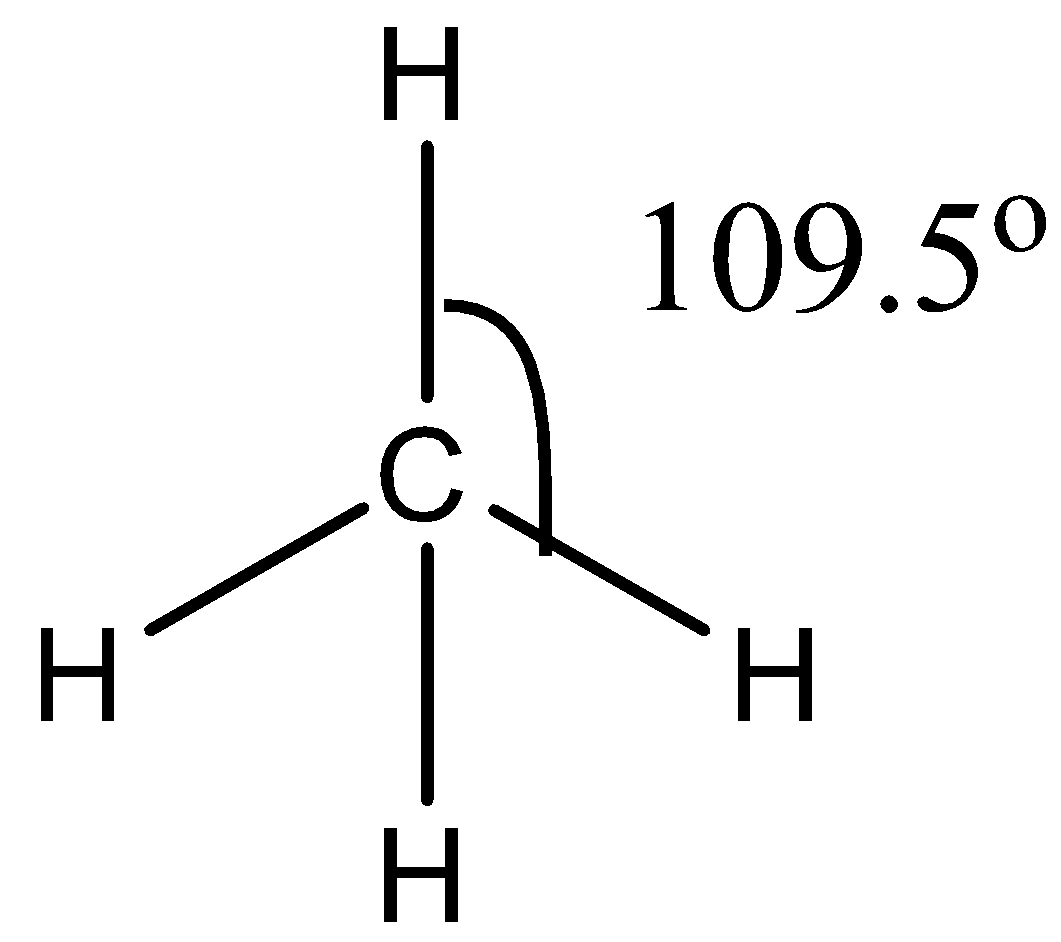
The ${{H}_{2}}O$ molecule has 2 bond pairs and 2 lone pairs. Due to the presence of lone pairs, the bond pairs will experience repulsion due to the bond pairs will come closer to each other. Due to this repulsion of lone pairs, the bond between bond angles becomes ${{104.5}^{\circ }}$. The structure of ${{H}_{2}}O$ is given below:

So, the bond angle of ${{H}_{2}}O$ decreases.
So, the correct answer is “Option C”.
Note: Since the hybridization of carbon and oxygen in $C{{H}_{4}}$ and ${{H}_{2}}O$ respectively same, i.e. $s{{p}^{3}}$. The structure of $C{{H}_{4}}$is tetrahedral. But the structure of ${{H}_{2}}O$ is bent or V-shaped due to the presence of 2 lone pairs.
Complete step by step answer:
The bond angle of a molecule tells the direction of the bonds.
When the molecule has a symmetric structure, i.e., they have only bond pairs. The bond angle can easily be determined. According to the hybridization of the molecule, the bond angle of the molecule can be predicted. If the molecule has $sp$ hybridization the bond angle is ${{180}^{\circ }}$, if the molecule has $s{{p}^{2}}$ hybridization, the bond angle is ${{120}^{\circ }}$, if the molecule has $s{{p}^{3}}$ hybridization the bond angle is ${{109.5}^{\circ }}$, etc.
The $C{{H}_{4}}$ molecule has a symmetric structure because it has four bond pairs. The hybridization of carbon in $C{{H}_{4}}$ is $s{{p}^{3}}$. The bond angle between the atoms is ${{109.5}^{\circ }}$. The structure of $C{{H}_{4}}$ is given below:

The ${{H}_{2}}O$ molecule has 2 bond pairs and 2 lone pairs. Due to the presence of lone pairs, the bond pairs will experience repulsion due to the bond pairs will come closer to each other. Due to this repulsion of lone pairs, the bond between bond angles becomes ${{104.5}^{\circ }}$. The structure of ${{H}_{2}}O$ is given below:

So, the bond angle of ${{H}_{2}}O$ decreases.
So, the correct answer is “Option C”.
Note: Since the hybridization of carbon and oxygen in $C{{H}_{4}}$ and ${{H}_{2}}O$ respectively same, i.e. $s{{p}^{3}}$. The structure of $C{{H}_{4}}$is tetrahedral. But the structure of ${{H}_{2}}O$ is bent or V-shaped due to the presence of 2 lone pairs.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




