
The Hinsberg test of a compound, ${{C}_{5}}{{H}_{14}}{{N}_{2}}$ produces a solid that is insoluble in 10% NaOH. This solid derivative dissolves in 10% aqueous sulphuric acid. Which of the following would best describe these facts?
(A) $N{{H}_{2}}C{{H}_{2}}C{{H}_{2}}N{{\left( C{{H}_{3}} \right)}_{2}}$
(B) ${{\left( C{{H}_{3}} \right)}_{2}}NC{{H}_{2}}C{{H}_{2}}NHC{{H}_{3}}$
(C) $N{{H}_{2}}C{{H}_{2}}C{{\left( C{{H}_{3}} \right)}_{2}}C{{H}_{2}}N{{H}_{2}}$
(D) ${{\left( C{{H}_{3}} \right)}_{2}}NC{{H}_{2}}N{{\left( C{{H}_{3}} \right)}_{2}}$
Answer
570.9k+ views
Hint: Heisenberg’s test is used to differentiate between primary amine, secondary amine and tertiary amine.
The reagent used for this reaction is phenyl sulfonyl chloride which can be prepared at the time of the reaction and can be a compound or a mixture of compounds.
Complete answer:
Let us discuss the Heisenberg’s test in detail,
This test is primarily used to differentiate within primary, secondary and tertiary amines. The amines here, acts as a nucleophile which attacks an electrophile i.e. Heisenberg’s reagent (benzene sulphonyl chloride).
Primary amine-
When Heisenberg’s reagent reacts with primary amine it gives a sulphonamide product which is soluble in alkali.
Secondary amine-
The reaction of Heisenberg’s reagent with secondary amine gives a sulphonamide which is insoluble in alkali but is water-soluble in the acid.
Tertiary amine-
There is no reaction in between tertiary amine and Heisenberg’s reagent.
Thus, this test is used for the differentiation of amines on the basis of the solubility of the obtained product in alkali.
Illustration-
In the given illustration we can see that,
Option (A) and (C) are primary amines; option (D) is tertiary amine. Hence, in accordance with the above given explanation only option (B) will satisfy the criteria as it is a secondary amine.
We can easily specify this by the reactions as follows,
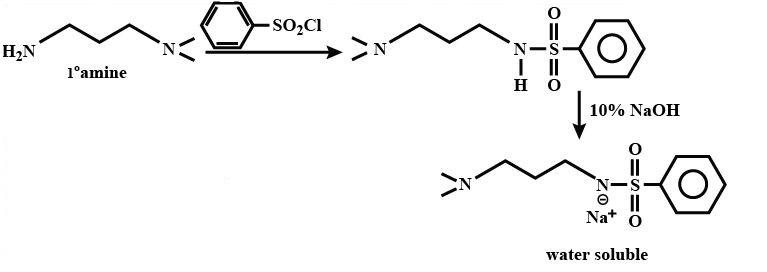

Therefore, option (B) is correct.
Note:
The terms used to classify amines as primary, secondary and tertiary are derived in very different form than that of other classifications as of alcohols or alkyl halides. During the classification of amines, these terms are used to refer to the number of alkyl substitutes directly bonded to N-atom.
The reagent used for this reaction is phenyl sulfonyl chloride which can be prepared at the time of the reaction and can be a compound or a mixture of compounds.
Complete answer:
Let us discuss the Heisenberg’s test in detail,
This test is primarily used to differentiate within primary, secondary and tertiary amines. The amines here, acts as a nucleophile which attacks an electrophile i.e. Heisenberg’s reagent (benzene sulphonyl chloride).
Primary amine-
When Heisenberg’s reagent reacts with primary amine it gives a sulphonamide product which is soluble in alkali.
Secondary amine-
The reaction of Heisenberg’s reagent with secondary amine gives a sulphonamide which is insoluble in alkali but is water-soluble in the acid.
Tertiary amine-
There is no reaction in between tertiary amine and Heisenberg’s reagent.
Thus, this test is used for the differentiation of amines on the basis of the solubility of the obtained product in alkali.
Illustration-
In the given illustration we can see that,
Option (A) and (C) are primary amines; option (D) is tertiary amine. Hence, in accordance with the above given explanation only option (B) will satisfy the criteria as it is a secondary amine.
We can easily specify this by the reactions as follows,


Therefore, option (B) is correct.
Note:
The terms used to classify amines as primary, secondary and tertiary are derived in very different form than that of other classifications as of alcohols or alkyl halides. During the classification of amines, these terms are used to refer to the number of alkyl substitutes directly bonded to N-atom.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




